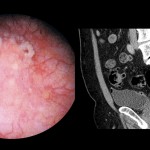
Video: Treating intrinsic sphincter deficiency with myofibre implantation
Periurethral skeletal myofibre implantation in patients with urinary incontinence and intrinsic sphincter deficiency: a phase I clinical trial
René Yiou1, Jean-Yves Hogrel8, Catherine-Marie Loche2, François-Jerome Authier3, Philippe Lecorvoisier7, Pauline Jouany4, Françoise Roudot-Thoraval5 and Jean-Pascal Lefaucheur6
1Hôpital Henri Mondor, Service d’Urologie and CRCDC, 2Hôpital Albert Chenevier, Service de Médecine Physique et de Réadaptation, 3Hôpital Henri Mondor, Département de Pathologie, 4Hôpital Henri Mondor, Unité de Recherche Clinique (URC), Pôle Recherche Clinique et Santé Publique, 5Hôpital Henri Mondor, Département de Santé Publique, Pôle Recherche Clinique et Santé Publique, UPEC, 6Hôpital Henri Mondor, Service de Physiologie – Explorations Fonctionnelles, Université Paris-Est, Faculté de Médecine, APHP, 7Hôpital Henri Mondor, INSERM, Créteil, and 8Université Paris 6, UMR S974, INSERM U974, CNRS UMR 7215, GH Pitié-Salpêtrière, Institut de Myologie, Paris, France
This open-label nonrandomized phase I clinical trial was registered on clinicalTrials.gov (#NCT00472069)
OBJECTIVES
• To assess the safety of periurethral myofibre implantation in patients with urinary incontinence due to intrinsic sphincter deficiency (ISD).
• To assess the resulting myogenic process and effects on urinary continence.
PATIENTS AND METHODS
• An open-label non-randomised phase I clinical trial was conducted in five men and five women with ISD (mean age, 62.5 years).
• A free muscle strip from the patient’s gracilis muscle was implanted around the urethra as a means to deliver locally myofibres and muscle precursor cells (MPCs).
• Patients were assessed for collection formation and incomplete bladder emptying.
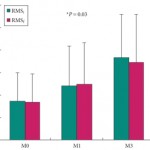
• The maximum urethral closure pressure (MUCP) and concomitant periurethral electromyographic (EMG) activity were recorded before surgery and 1 and 3 months after surgery. Continence was assessed using the 24-h pad test and self-completed questionnaires, for 12 months.
RESULTS
• There were no serious side-effects.
• Continence improved significantly during the 12-month follow-up in four of the five women, including two who recovered normal continence. In the women, MUCP increased two-fold and de novo EMG periurethral activity was recorded. In the men, MUCP and EMG recordings showed similar improvements but the effect on continence was moderate.
• The few patients enrolled could affect these results.
CONCLUSIONS
• This is the first report of a one-step procedure for transferring autologous MPCs via myofibre implantation in patients with ISD.
• EMG and urodynamic assessments showed improvement of periurethral muscle activity.
• Further work is needed to confirm and improve the therapeutic efficiency of this procedure.