Video: IFES to manage non-neuropathic UAB in children
Transcutaneous interferential electrical stimulation for the management of non-neuropathic underactive bladder in children: a randomised clinical trial
Objectives
To assess the efficacy of transcutaneous interferential electrical stimulation (IFES) and urotherapy in the management of non-neuropathic underactive bladder (UAB) in children with voiding dysfunction.
Patients and Methods
In all, 36 children with UAB without neuropathic disease [15 boys, 21 girls; mean (sd) age 8.9 (2.6) years] were enrolled and then randomly allocated to two equal treatment groups comprising IFES and control groups. The control group underwent only standard urotherapy comprising diet, hydration, scheduled voiding, toilet training, and pelvic floor and abdominal muscles relaxation. Children in the IFES group likewise underwent standard urotherapy and also received IFES. Children in both groups underwent a 15-session treatment programme twice a week. A complete voiding and bowel habit diary was completed by parents before, after treatment, and 1 year later. Bladder ultrasound and uroflowmetry/electromyography were performed before, at the end of treatment course, and at the 1-year follow-up.
Results
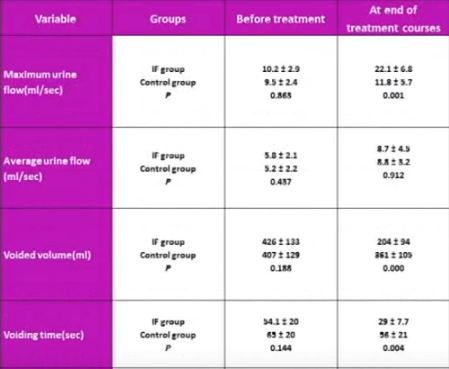
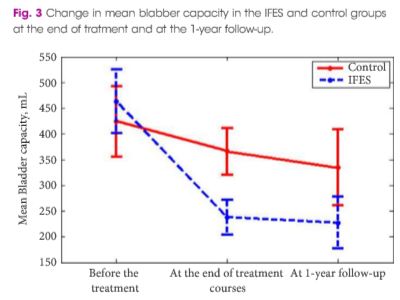
The mean (sd) number of voiding episodes before treatment was 2.6 (1) and 2.7 (0.76) times/day in the IFES and control groups, respectively, which significantly increased after IFES therapy in IFES group, compared with only standard urotherapy in the control group [6.3 (1.4) vs 4.7 (1.3) times/day, P < 0.002). The mean (sd) bladder capacity before treatment was 424 (123) and 463 (121) mL in the control and IFES groups, respectively, which decreased significantly at 1 year after treatment in the IFES group compared with the controls, at 227 (86) vs 344 (127) mL (P < 0.01). Maximum urine flow increased and voiding time decreased significantly in the IFES group compared with controls at the end of treatment sessions and 1 year later (P < 0.05). All the children had abnormal flow curves at the beginning of the study. The flow curve became normal in 14/18 (77%) of the children in the IFES group and six of 18 (33%) in the control group by the end of follow-up (P < 0.007). At the end of the treatment course, night-time wetting was improved in all children who had this symptom before the treatment in the IFES group (P < 0.01).
Conclusion
Combining IFES and urotherapy is a safe and effective therapy in the management of children with UAB.