Article of the week: Aquablation for benign prostatic hyperplasia in large prostates: 6‐month results from the WATER II trial
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
We invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Aquablation for benign prostatic hyperplasia in large prostates (80–150 mL): 6‐month results from the WATER II trial
Mihir Desai*, Mo Bidair†, Kevin C. Zorn‡, Andrew Trainer§, Andrew Arther§, Eugene Kramolowsky¶, Leo Doumanian*, Dean Elterman**, Ronald P. Kaufman Jr.††, James Lingeman‡‡, Amy Krambeck‡‡, Gregg Eure§§, Gopal Badlani¶¶, Mark Plante***, Edward Uchio†††, Greg Gin†††, Larry Goldenberg‡‡‡, Ryan Paterson‡‡‡, Alan So‡‡‡, Mitch Humphreys§§§, Claus Roehrborn¶¶¶, Steven Kaplan****, Jay Motola**** and Naeem Bhojani‡
*Institute of Urology, University of Southern California, Los Angeles, †San Diego Clinical Trials, San Diego, CA, USA, ‡University of Montreal Hospital Center, Université de Montréal, Montréal, QC, Canada, §Adult Pediatric Urology and Urogynecology, P.C., Omaha, NE, ¶Virginia Urology, Richmond, VA, USA, **University of Toronto – University HealthNetwork, Toronto, ON, Canada, ††Albany Medical College, Albany, NY, ‡‡Indiana University Health Physicians, Indianapolis, IN, §§Urology of Virginia, Virginia Beach, VA, ¶¶Wake Forest School of Medicine, Winston-Salem, NC, ***University of Vermont Medical Center, Burlington, VT, †††VA Long Beach Healthcare System, Long Beach, CA, USA, ‡‡‡University of British Columbia, Vancouver, BC, Canada, §§§Mayo Clinic Arizona, Scottsdale, AZ, ¶¶¶UT Southwestern Medical Center, Department of Urology, University of Texas Southwestern, Dallas, TX, and ****Icahn School of Medicine at Mount Sinai, New York, NY, USA
Abstract
Objective
To present 6‐month safety and effectiveness data from a multicentre prospective study of aquablation in men with lower urinary tract symptoms (LUTS) attributable to benign prostatic hyperplasia (BPH) with prostate volumes between 80 and 150 mL.
Methods
Between September and December 2017, 101 men with LUTS attributable to BPH were prospectively enrolled at 16 centers in Canada and the USA.
Results
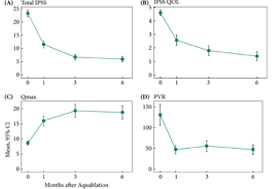
The mean prostate volume was 107 mL. The mean length of hospital stay after the aquablation procedure was 1.6 days (range: same day to 6 days). The primary safety endpoint (Clavien–Dindo grade 2 or higher or any grade 1 event resulting in persistent disability) at 3 months occurred in 45.5% of men, which met the study design goal of < 65% (P < 0.001). At 6 months, 22% of the patients had experienced a Clavien–Dindo grade 2, 14% a grade 3 and 5% a grade 4 adverse event. Bleeding complications requiring intervention and/or transfusion were recorded in eight patients prior to discharge and in six patients after discharge. The mean International Prostate Symptom Score improved from 23.2 ± 6.3 at baseline to 6.7 ± 5.1 at 3 months, meeting the study’s primary efficacy endpoint goal (P < 0.001). The maximum urinary flow rate increased from 8.7 to 18.8 mL/s (P < 0.001) and post‐void residual urine volume decreased from 131 at baseline to 47 at 6 months (P < 0.0001). At 6 months, prostate‐specific antigen concentration reduced from 7.1 ± 5.9 ng/mL at baseline to 4.0 ± 3.9 ng/mL, a 44% reduction.
Conclusions
Aquablation is safe and effective in treating men with larger prostates (80–150 mL), without significant increase in procedure or resection time.