Video abstract: clinical trial protocol of the LUNAR study
LUNAR: a randomized Phase 2 study of 177Lutetium-PSMA Neoadjuvant to Ablative Radiotherapy for Oligorecurrent Prostate Cancer (clinical trial protocol)
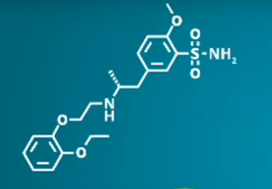
The aim of this study is to assess the efficacy of 177Lu-PNT2002, a novel radiolabelled small molecule that binds with high affinity to prostate-specific membrane antigen, in combination with stereotactic body radiotherapy (SBRT) to all sites of metastasis, vs SBRT alone, in men with oligorecurrent metastatic hormone-sensitive prostate cancer.
Ting Martin Ma, Johannes Czernin, Carol Felix, Rejah Alano, Holly Wilhalme, Luca Valle, Michael L. Steinberg, Magnus Dahlbom, Robert E. Reiter, Matthew B. Rettig, Minsong Cao, Jeremie Calais, Amar U. Kishan