Improved early detection of prostate cancer has led to an increased incidence of this disease, and an increase in the number of patients undergoing radical prostatectomy (RP). The rate of post-prostatectomy incontinence (PPI) is difficult to determine because of the varying definitions of incontinence, but approximately one in five men require the use of pads in the long term after RP. Incontinence has a significant negative impact on quality of life, and remains many men’s greatest fear, especially for the one in four who present at the age of <65 years. While significant advancements have been made in prostate cancer treatment, strong evidence for the optimum management of PPI remains lacking. Most guidelines are based on grade B or C recommendation and many questions about its surgical management remain unanswered.
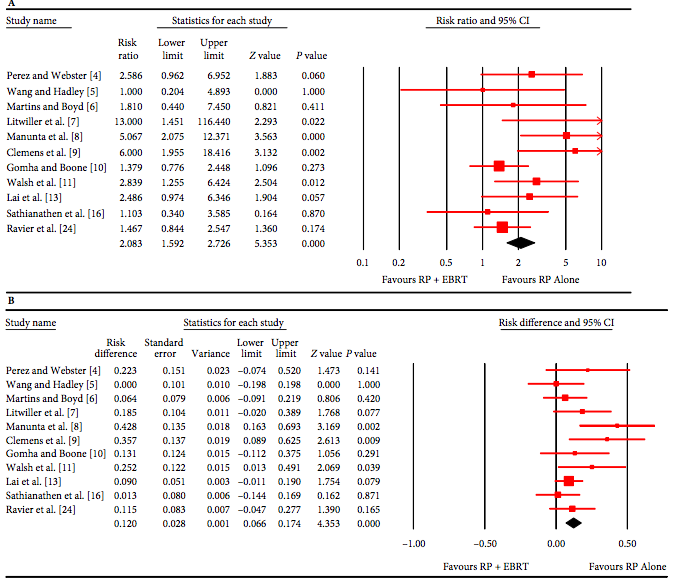
The artificial urinary sphincter (AUS) has stood the test of time and has long been considered the ‘gold standard’ treatment for PPI, especially for those with moderate to severe incontinence. The quoted success rates achieved with this device vary from study to study based on the varying definition of ‘dry’. The use of radiotherapy (RT) after prostatectomy is generally considered to have a negative impact on its efficacy and revision rate, although some data have been conflicting. In this month’s BJUI, Bates et al. [1] present a timely and well-structured systematic review and meta-analysis of AUS placement after RP and RT. By analysing pooled results, the authors set out to clarify the effect of RT on AUS efficacy and outcomes. In total, 1886 patients from 15 studies published between 1989 and 2014 were included in the meta-analysis, including 14 studies assessing surgical revision and 11 looking at persistent urinary incontinence. No randomized controlled trials were available for analysis. Retrospective reporting and a lack of standardized postoperative validated assessments were a weakness of individual studies, and efforts to limit the effects of study heterogeneity and risk of bias were made using statistical models. The revision rate after a mean follow-up of 38.4 months was significantly higher in irradiated vs. non-irradiated men (mean 37.3 vs 19.8%; P < 0.007); the risk ratio was 1.56 and number needed to harm was 4 (i.e. one surgical revision for every four AUS devices implanted in irradiated men). Infection/erosion and urethral atrophy accounted for approximately half and one-third of all revisions respectively. Persistent urinary incontinence was also more than twice as likely in irradiated vs non-irradiated men (29.5 vs 12.1%; P = 0.003; risk ratio 2.08, number needed to harm 9).
This study highlights the significant negative impact of RT after RP on functional outcomes and its treatment. This is particularly important considering that approximately one-third of patients will require adjuvant or salvage radiotherapy at some stage after RP. The development of incontinence after RT is primarily attributable to the negative effect of radiation on bladder and urethral tissue. Unlike outcomes with regard to erectile function, the type of primary surgery performed (open vs robotic) does not appear to have any significant impact on PPI [2]. Timing of RT also does not seem to affect function, with similar rates of incontinence reported for early (<6 months after RP) vs late (>6 months after RP) irradiation reported 3 years after RT (24.5 vs 23.3%, respectively; P = 0.79) [3].
New devices, such as the male sling, have increased the options for PPI treatment. Male slings have achieved popularity because of their safety, relative ease of insertion and patients’ strong desire to void naturally without fiddling with pumps. Kumar et al. [4] reported that one in four men who were recommended an AUS as the best option by their surgeon chose a sling; 92% who were offered either also opted against the gold standard AUS. Slings, however, have not fared well in patients with severe incontinence or those who have undergone RT. Pooled analysis of the AdVance® sling reported ‘success’ rates of 56 and 54%, respectively, in these scenarios, compared with a mean overall ‘success’ rate of 75% [5]. Reported success, however, does not equate to being ‘dry’, as reported in many AUS studies, and this lack of uniformity in describing outcomes prevents adequate clarity when comparing different devices. Despite the lower success rate after RT, slings, unlike the AUS, do not appear to have any additional complications in this setting [1, 6], and sling failure does not appear to prejudice subsequent AUS placement [7].
To date, no randomized controlled trial has directly compared efficacy of the newer slings with the AUS. Well-designed trials, with standardized protocols and uniform long-term assessments of outcome, including complications and quality of life, are required to clarify their place in managing PPI. Current randomized controlled trials are evaluating these devices prospectively, and will provide much needed level 1 evidence in this field. The most interesting of these is the MASTER trial (Male synthetic sling vs Artificial urinary Sphincter Trial). This multicentre UK randomized controlled trial is for men with incontinence after prostate surgery for cancer or benign disease [8]. Patients of any age, with any level of incontinence are eligible, and previous RT is not an exclusion criterion. The trial aims to randomize 360 men and will also follow up 360 non-randomized men, and runs until 2019. This trial will help clarify the relative benefits of the devices by incontinence severity. It will also provide some prospective data on the effect of RT on outcomes, although the 2-year follow-up will be too short to evaluate this fully.
The question remains regarding which strategy is the best for post-prostatectomy irradiated patients. Until the results of good quality trials are available, the jury is out. The AUS remains the gold standard in this setting, for now. For patients with mild to moderate incontinence, the sling is an option, and offers some advantages, but offers a lower overall chance of becoming pad free. Patients must be carefully counselled about the risk/benefit of this approach compared with an AUS. Results of the MASTER trial will help better define management of this subgroup. For moderate to severe incontinence, the AUS is the gold standard, albeit with an increased risk of failure and revision. The present meta-analysis arms the clinician with much needed data to quantify the relative risk of complications and adverse outcomes in this setting, and will allow better counselling and management of patient’s expectations.
Majid Shabbir
Department of Urology, Guy’s Hospital, London, UK
References
1 Bates A, Martin R, Terry T. Complications following artificial urinary sphincter placement after radical prostatectomy and radiotherapy: a metaanalysis. BJU Int 2015; 116: 623–33
2 Haglind E, Carlsson S, Stranne J et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol 2015. doi: 10.1016/j.eururo. 2015.02.029. [Epub ahead of print]
3 Sowerby RJ, Gani J, Yim H. Long-term complications in men who have early or late radiotherapy after radical prostatectomy. Can Urol Assoc J 2014; 8: 253–8.
4 Kumar A, Litt ER, Ballert KN, Nitti VW. Artificial urinary sphincter versus male sling for post-prostatectomy incontinence-what do patients choose? J Urol 2009; 181: 1231–5.
5 Van Bruwaene S, Van der Aa F, De Ridder D. Review: the use of sling versus sphincter in post-prostatectomy urinary incontinence. BJU Int 2015; 116: 330–42
6 Zuckerman JM, Tisdale B, McCammon K. AdVance male sling in irradiated patients with stress urinary incontinence. Can J Urol 2011; 18: 6013–7.
7 Lentz AC, Peterson AC, Webster GD. Outcomes following artificial sphincter implantation after prior unsuccessful male sling. J Urol 2012; 187: 2149–53.
8 Abrams P. Male synthetic sling versus Artificial urinary Sphincter Trial for men with urodynamic stress incontinence after prostate surgery: Evaluation by Randomised controlled trial (MASTER), 2014. Available at: www.controlled-trials.com/ISRCTN49212975/MASTER. Accessed May 2015