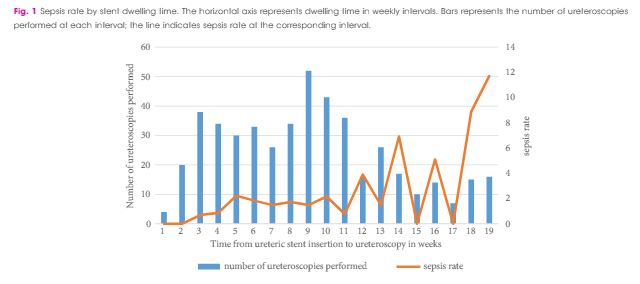
In this edition of the BJUI, Nevo et al. [1] report their retrospective review of 1256 patients who underwent ureteroscopy (URS)/flexible ureterorenoscopy (FURS) for stone disease and identified an overall sepsis rate of 2.8% within 48 h of surgery. About half of the cohort had a previously placed JJ stent, and the key finding of the study was the association between this and postoperative sepsis. In particular, the risk of sepsis in unstented patients was 1.2%, compared with 4.7% in those with a stent, such that overall, 80% of the patients who developed sepsis had a prior JJ stent in situ. Furthermore, this risk increased cumulatively with longer stent dwell-time before definitive surgery, increasing the risk of sepsis to 9.2% in patients who had a stent in situ for >3 months before the treatment of their stone.
Pre-stenting before ureteroscopic stone treatment can be for ‘absolute’ reasons (e.g. achieving ureteric drainage following presentation with an obstructed/infected kidney, or for an inaccessible ureter during the initial attempt at ureteroscopic stone treatment) or ‘relative’ reasons (e.g. emergency pain relief from ureteric colic if stone clearance cannot be offered immediately, or strategically inserted to allow passive ureteric dilatation to facilitate a subsequent definitive procedure). Data from the Clinical Research Office of the Endourological Society (CROES) URS Global Study showed that pre-stenting occurred in 36.4% of patients with rena l stones, and was associated with an increased stone-free rate (SFR), with a small but significant decrease in intraoperative complications. Pre-stenting was less common in ureteric stone management (11.9% of 8189 patients) and showed no difference in the SFR or complications, but was associated with a shorter length of stay [2]. Similarly, Jessen et al. [3] have reported that pre-stenting conferred a significant improvement in SFR for renal stones (83% vs 60%) but not for ureteric stones (94% vs 90%), although complications were reduced for pre-stented vs unstented patients in both renal stones (8.7% vs 19.4%) and ureteric stones (3.1% vs 10.7%) in that study. The advantage of pre-stenting appears to be greatest for larger stones: as a consequence of ureteric dilatation, and therefore improved renal access, in patients with stones >1 cm in whom a multi-phased approach was anticipated, Chu et al. [4] found that pre-stenting significantly reduced the operative time of the first URS, as well as the total operative time to stone clearance, including the need to re-operate at all in some patients.
The study in this edition of the BJUI [1] has shown that these advantages must be balanced against the increased risk of postoperative sepsis in patients with a pre-placed JJ stent, particularly in cases where the stent has been in situ for >1 month. The ability to identify patients who are at greater risk of post-URS/FURS infections is clearly useful: female gender, diabetes mellitus, ischaemic heart disease, an American Society of Anesthesiologists (ASA) score of ≥II, a large-volume stone burden, and same-session bilateral URS, have already been established as significant risk factors for postoperative infection [5, 6]. In addition, preoperative infections, either as a positive midstream specimen of urine (MSU) or previous sepsis also increase the risk of postoperative infectious complications. Specifically, Blackmur et al. [6]reported that patients with a positive preoperative MSU were about five-times more likely to have postoperative urosepsis, even if they had been treated with an appropriate course of antibiotics before their stone surgery. Consistent with this, Youssef et al. [7] reported that the complication rate in patients undergoing URS after previous sepsis increased to 20% compared to 7% in matched non-septic controls, with an associated increased length of stay and duration of postoperative antibiotics.
Taken together, these studies suggest that pre-stenting may have value in improving SFR, total operation time and reducing complication rates for large renal stones (i.e. >1 cm), but offers less advantage to stone clearance rates or complications in ureteric stones. Given the findings of increased risk of sepsis with stent-dwell time (increasing from 2.2% at 30 days, to 4.9% at 60 days, 5.5% at 90 days and 9.2% for >90 days) the authors recommendation ‘to keep stent dwelling time as short as possible’, is both practically beneficial to the patient and evidence-based. Furthermore, in addition to an awareness of the recognised risk factors for postoperative sepsis mentioned above, patients who have had stents inserted for prior sepsis, patients with positive preoperative MSU (even if treated), and patients with prolonged stent duration before definitive treatment should be counselled of the greater risk of postoperative sepsis, and watched cautiously in the early postoperative period.
In this study [1], patients with a stent in situ for <1 month had a similar risk of UTI to unstented patients. It would therefore seem reasonable to conclude that patients who have a stent inserted, especially for more ‘relative’ reasons to facilitate future surgery, should be scheduled for their definitive procedure within a month to achieve the benefits of pre-stenting, whilst minimising the potential for postoperative septic complications that Nevo et al. [1] have highlighted.
Daron Smith
Institute of Urology, University College Hospital, London, UK
References