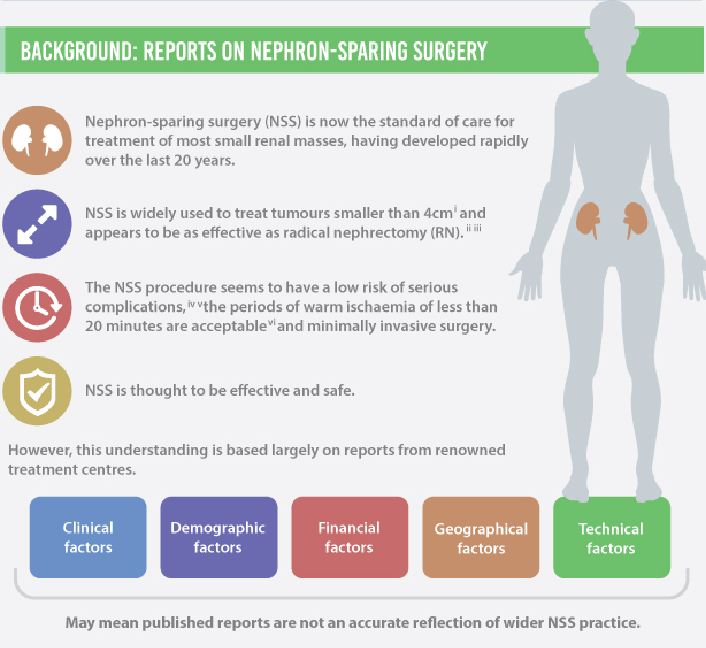
The perceived wisdom that a small enhancing mass in the kidney represents a surgical lesion that automatically requires excision without the need for a preoperative biopsy has been challenged by Fernando et al. [1] in this issue of BJUI.
The authors are to be congratulated in bringing these data to publication to provoke debate on the treatment paradigm for small renal masses (SRMs) by reviewing nationally collected data on the main therapeutic surgical option: nephron-sparing surgery. As anyone who has attended a renal multidisciplinary meeting can testify, the predominant presentation of renal cancer is the incidentally detected SRM, often in elderly patients with significant comorbidity.
As the authors emphasize, these data are unique in representing a national picture encompassing both high- and low-volume centres, as opposed to the majority of the studies in the literature, which report data from high-volume tertiary referral centres.
Drawing conclusions from data requires a clear understanding of the source and quality. Most importantly, as these data only refer to patients undergoing nephron-sparing surgery, we need to be cautious about extrapolating to infer information on the management of SRMs in general.
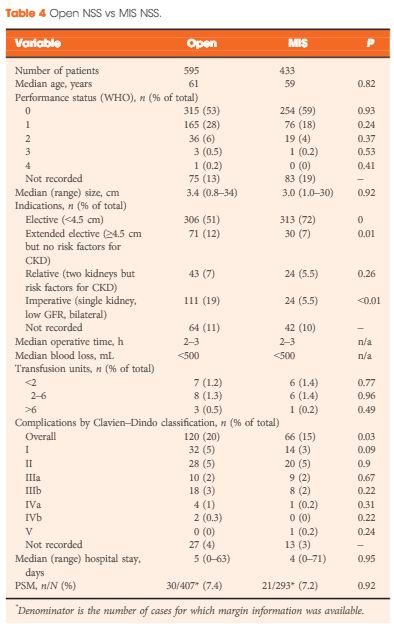
For instance, a striking finding of the present study is the high incidence of benign lesions in the younger age groups. We have no knowledge of the numbers of patients with SRMs within the study period who had biopsy-proven benign disease and thus avoided surgery. It is probable that the true incidence of benign disease would be even higher if these cases had been recorded and included in the analysis.
An inherent difficulty with self-reported data is the issue of compliance, and this is clearly evident in the present study, with, for example, almost a third of cases missing data on surgical margin results. It would perhaps be helpful for future audits if the BAUS dataset had a clear definition of positive surgical margin in recognition of the surgical drift to enucleation rather than excision with a margin of renal parenchyma.
The variation in caseload between reporting centres raises important questions, as does the finding that two fifths of patients with T1a tumours underwent radical nephrectomies. As the authors concede, with the numbers involved and the absence of any measure of tumour complexity, it is difficult to draw firm conclusions; however, the study does highlight the need to examine this issue in future analyses and to consider including some form of renal scoring system in future audits.
Where do we go from here and what can we do with this information? First, we need to rethink our discussion with patients with SRMs. Can we justify performing major surgery with a one in 20 chance of a significant complication for a possible benign lesion without at least a pragmatic discussion of the role of renal biopsy with the patient? Indeed, one may argue, could it really be an ‘informed’ decision without it?
Second, we need to improve the quality of the data by encouraging robust data reporting, increasing the completion rate and considering adding data fields which will allow us to draw clearer conclusions on surgical margin and surgical outcome and volume relationships.
Third, we need to recognize that nephron-sparing surgery is only one component of the management of SRMs, which represents a major contemporary challenge in terms of health resources and, most importantly, in deciding the best treatment paradigm for our patients. If BAUS can carry out this audit, could we not extend this to all patients with SRMs, whether they have surgery, ablation or surveillance, and establish greater clarity on these treatment methods?
Michael Aitchison, Consultant Urological Surgeon and Maxine Tran, Senior Lecturer in Renal Cancer Surgery and Honorary Consultant Urological Surgeon
Renal Cancer Service, Royal Free NHS Foundation Trust, London, UK
Reference