This case is the first reported case of acquired haemophilia that is solely attributed to an underlying bladder tumour.
Authors: Fitzpatrick J, Aboumarzouk O, Ahmad S, Byrne D, Nabi G. Ninewells Hospital, NHS Tayside, Dundee, DD1 9SY, UK
Corresponding Author: Omar M Aboumarzouk, Ninewells Hospital, NHS Tayside, Dundee, DD1 9SY, UK. E-mail: aboumarzouk@gmail.com
Abstract
Acquired haemophilia is a rare bleeding disorder caused by the development of autoantibodies to coagulation factor VIII (FVIII). The clinical presentation varies, ranging from mild bleeding to acute and life-threatening haemorrhage, with mortality approaching 22%. Around 50 percent of cases are idiopathic, however, it is also associated with several underlying conditions, including malignancy. We report a case of acquired haemophilia in an 80-year-old patient with transitional cell carcinoma of the bladder (grade G3pT1), who underwent transurethral resection of bladder tumour (TURBT). Unfortunately, five weeks after admission, he died after being treated with recombinant factor VIIa, rituximab, and undergoing multiple transurethral resections and washouts for recurrent blood clots within the bladder. The repeated recombinant factor VIIa and rituximab lead to the formation of a thick and adhesive clot in his bladder requiring these repeated procedures. This is the first reported case of acquired haemophilia that is solely attributed to an underlying bladder tumour.
Background
Acquired haemophilia (AH) is an autoimmune disease characterised by a deficiency in coagulation factor VIII (FVIII).[1,2] The pathophysiology of the disease can be explained by the acquisition of antibodies, which neutralise the procoagulant properties of FVIII, resulting in severe and often life-threatening haemorrhage.[3,4] AH tends to present later in life, with the mean age of onset at 65 years, and affects both genders equally.[2,3] In 50 percent of cases, there is an idiopathic aetiology, however, it is often associated with underlying conditions, including autoimmune diseases, solid tumours, lymphoproliferative disorders, drug hypersensitivity and pregnancy.[3,4,5,6] A laboratory diagnosis of AH is twofold; in the first instance, by a prolonged activated partial thromboplastin time (APTT), not corrected by incubation with normal plasma in the presence of a normal prothrombin time (PT), and secondly by reduced FVIII, with evidence of FVIII inhibitor measured by the Bethesda assay.[3,4] The principles of management are to control bleeding, primarily with recombinant factor VIIa (rFVIIa), and to introduce long-term eradication of autoantibodies by immunosuppression; this is often achieved by using a combination of steroids, cyclophosphamide, intravenous immunoglobulins and monoclonal antibodies.[1,3] Although acquired haemophilia is a rare condition, with an incidence of 1-4 per million/year, there is a significant morbidity associated with it; 90% of affected individuals will suffer a substantial bleed and up to 22% will not survive the episode.[1,4,7]
Case report
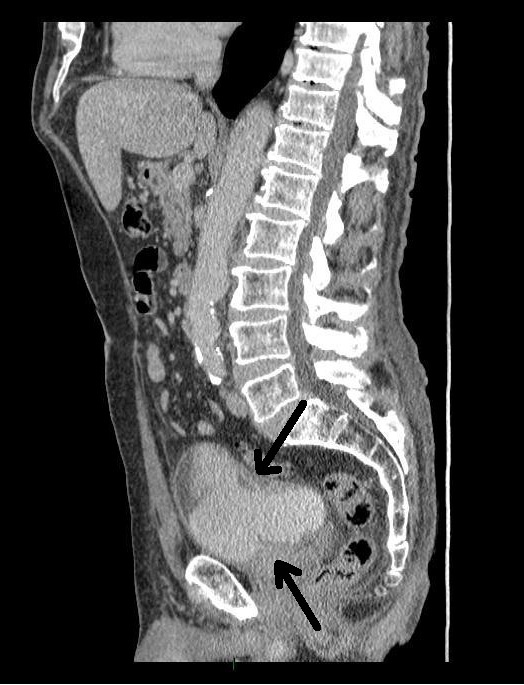
An 80 year-old male, with no significant past medical history, was referred to our urology department after presenting to his GP with frank haematuria. He subsequently underwent a CT scan (Image 1, 2) which showed three moderate-sized papillary lesions on the right lateral wall of the bladder. Flexible cystoscopy confirmed the diagnosis, subsequently shown to be a G3 pT1 tumour. The decision was for him to undergo a transurethral resection of bladder tumour (TURBT) and Mitomycin-C instillation.
Image 1. CT scan, coronal
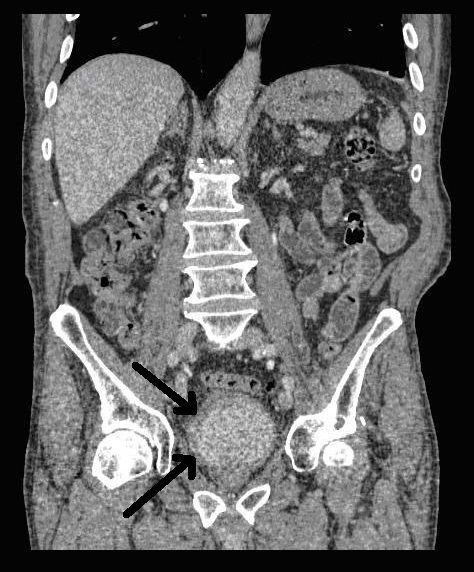
Image 2. CT scan, saggital
The following week he presented to the Accident &Emergency Department with chest pain. His ECG was normal and his pain rapidly settled. He was therefore discharged and advised to contact his GP if his symptoms persisted. The following day, he attended his GP again complaining of chest pain with shortness of breath and further episodes of haematuria. He was referred directly to the urology ward.
On admission, he was noted to have extensive bruising across his arms, abdomen and flanks. There was no history of traumatic injury, although the bruising had become more extensive over the past week.
Blood tests confirmed that he was anaemic, with a haemoglobin of 7.1 g/dL. His coagulation screen showed a prolonged APTT of 76.3 sec (normal range 22-30 sec) with a normal PT of 9.7 sec (normal range 9-12 sec). His Troponin T level was grossly elevated at 852 ng/L (normal range 0-13 ng/L), however, this was not due to myocardial infarction, as there was no significant rise in the Troponin T level at 12 hours.
In light of the abnormal coagulation results, a haematology consult was sought. A FVIII assay result of 1.2 u/dl (normal range 50-150 u/dl) and a Bethesda assay result of 17.3 Beth.U confirmed a diagnosis of acquired haemophilia. He was transfused three units of blood and was commenced on rFVIIa (NovoSeven) at 90 µg/kg and prednisolone at 1 mg/kg, with proton pump inhibitor (PPI) cover. Subsequent testing, including autoimmune screening, anti-lupus anticoagulant and viral serology was carried out to try to determine the underlying aetiology, however, these failed to yield a positive result. A renal ultrasound scan was also normal. It was concluded, therefore, that the most likely explanation for the acquired haemophilia was his bladder cancer.
The decision was made to bring forward the planned TURBT in the hope that this would lead to a resolution of the acquired haemophilia. It was performed with preoperative and postoperative rFVIIa cover. Postoperatively, there appeared to be an early response following removal of the bladder tumour; FVIII levels improved from 1.2 u/dl to 6.5 u/dl, although he did require further blood transfusion. The haematuria settled and the catheter removed the following day.
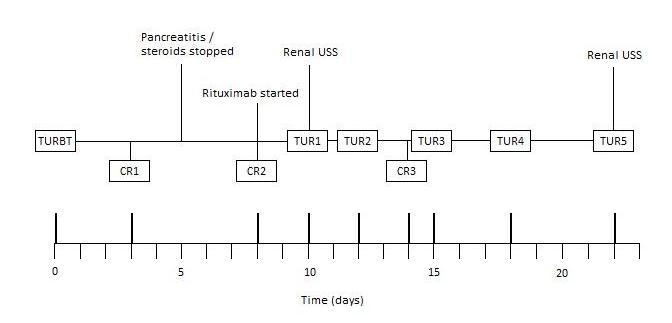
Unfortunately, three days later, he developed clot retention. This became a recurring problem throughout admission, requiring multiple three-way catheter insertions, irrigation and bladder washouts. The clinical course has been summarised in Figure 1.
Figure 1. Clinical course summary
He developed acute abdominal pain with associated nausea. Blood tests revealed an amylase of 1641 units per liter (U/L) and he was diagnosed with pancreatitis. With no history of alcohol excess and no gallstones identified on prior imaging, the underlying aetiology was believed to be steroid-induced and his prednisolone was subsequently stopped. Treatment with intravenous fluids was successful, although he required further blood transfusion and rFVIIa. Rituximab was started by the haematologists.
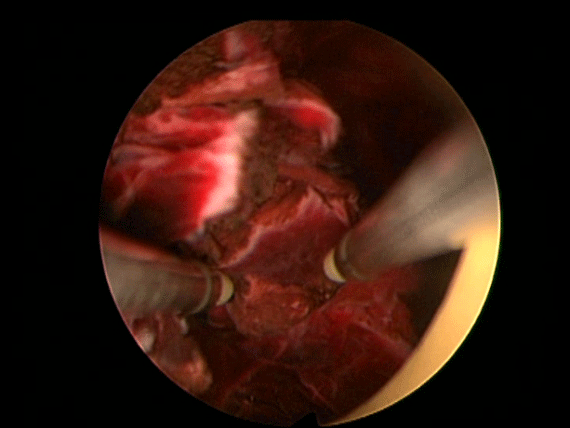
In order to further investigate the continuing haematuria, an ultrasound scan was arranged. This revealed a substantial clot within the bladder, approximately 6 x 6 cm in size. He was taken to theatre and a transurethral evacuation of the clot was carried out. The procedure was technically difficult and took around three hours to perform. The clot was thick, adhesive and organised, with resected clot reattaching to the bladder wall (Image 3, 4). Unfortunately, only part of the clot could be successfully evacuated.
Image 3.
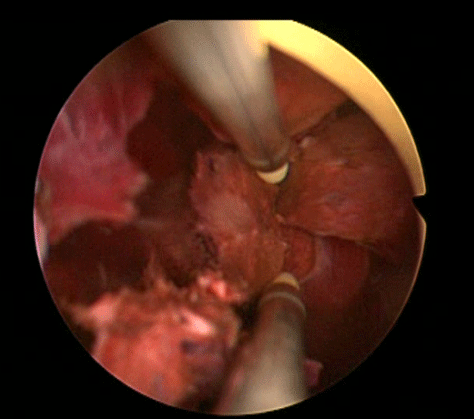
Image 4.
Two days later, he was taken back to theatre for a second attempt to evacuate the remainder of the clot. Rituximab and rFVIIa were given preoperatively. Once again, the resection was difficult and prolonged; after four hours of resection, only a small portion had been removed. The procedure was stopped, as the patient was becoming hypothermic and acidotic. Two units of blood were transfused.
Third and fourth evacuations were arranged to remove the residual clot. The third took approximately two hours. Warm irrigation fluid was used and some mobile organised clot remained at the end of the procedure. On the fourth attempt, the formed clot was cleared entirely. As with the previous resections, preoperative rVIIa and postoperative blood transfusion were required. It was encouraging to note that the APTT was improving gradually on rituximab and was now 68 sec.
As the APTT continued to improve, we decided to take a more conservative approach and see if it would correct with rituximab alone. The APTT improved further to 54 sec, however, repeat FVIII assay showed only a mild improvement (2.1 u/dL) and FVIII inhibitor was still present (17.8 Beth.U).
Unfortunately, over the next few days, the patient’s clinical condition deteriorated. A further seven units of blood were required during this time and his renal function worsened. An ultrasound scan revealed that, once again, there was a large formed clot in the bladder. A fifth cystoscopy was arranged, which confirmed a large organised clot occupying the whole bladder and blocking both ureteric orifices. The prognosis was poor and treatment had now become palliative. The clot was left in the hope that it might act to tamponade the bladder, however, the resulting obstructive uropathy meant that renal failure and hyperkalaemia developed; he passed away shortly after.
Discussion
The aetiology of AH is idiopathic in about 50 percent of cases, however, it is often associated with a variety of clinical conditions such as autoimmune disease (asthma, rheumatoid arthritis, systemic lupus erythematosus), solid tumours, lymphoproliferative disorders, drug hypersensitivity and pregnancy.[3,4,5,6] AH is associated with underlying malignancies in 7-15 percent of cases and is more prevalent in solid tumours than in haematological malignancies.[1,3,8,9] Reitter et al collated all published cases of cancer-associated FVIII autoantibodies from 1950-2010.[10] Although their analysis primarily targeted those cases in which FVIII autoantibodies developed following cancer surgery, their initial literature search also revealed those cases where AH had been diagnosed prior to surgery. Of the 57 cases uncovered, 32% (18/57) were associated with urological malignancies (14 prostate, 4 kidney), with prostate cancer the most prevalent amongst all cancer types.[10] Interestingly, there were no reported cases of AH secondary to bladder cancer; the only case found was in a patient who had developed FVIII autoantibodies following surgical treatment of a bladder tumour.[10]
AH due to underlying bladder cancer is extremely rare; a search of the literature only yielded two published cases, however, both have been multifactorial in their aetiologies.[11,12] In 2005, Kreuter et al reported AH in a patient with gram-negative urosepsis following resection of a rectal tumour, who underwent resection of a bladder tumour 6 months later.[11] Kato et al described AH in a patient with concomitant bladder and renal tumours.[12] To our knowledge, therefore, this is the first reported case of AH that can be attributed solely to bladder cancer.
The treatment of acquired haemophilia has been a much debated topic, in part due to its rarity and clinical diversity, with management plans often determined by the individual experiences of haematologists and on a case-by-case basis. As a result, data on the efficacy of anti-haemorrhage drugs is often retrospective.[1,13] There exists, however, generally accepted principles, primarily (1) controlling bleeding episodes, (2) avoiding invasive procedures that may precipitate further bleeding, (3) eradicating FVIII inhibitor through immunosuppression and (4) treating any underlying disease. [1,2,3,5]
Controlling bleeding episodes
The two main first-line agents that are licensed for the treatment of AH are rFVIIa (NovoSeven) and activated prothrombin complex concentrate (aPCC) (FEIBA; Factor VII inhibitor bypassing activity). [1,14] In our case, rFVIIa was adopted as the primary agent, using the standard recommended dose of 90 µg/kg.1 Such bypassing agents, however, are associated with an increased risk of arterial thrombosis, often in patients with underlying cardiovascular risk factors. A literature review demonstrated this in 7% of patients treated with rFVIIa.[14] The prothrombotic properties of bypassing agents are clearly useful in the cessation of bleeding episodes; however, it has been this very effect that has complicated our management. On one hand, rVIIa has been able to control bleeding and haematuria, however, it has also predisposed to the formation of further bladder clots, leading to recurrent catheter blockages and multiple clot resections.
Eradication of FVIII inhibitor
There is no definitive optimal regimen for inhibitor eradication. The most commonly adopted strategy is to use either corticosteroids alone or corticosteroids in combination with cyclophosphamide, with a lack of evidence supporting the choice of one over the other.[1,2,6] Previous studies have suggested that 70-80% of patients will achieve remission on either regimen; however, different durations of therapy were used in each.[13,15] In our case, prednisolone alone was used at 1 mg/kg; this is the dose most widely recommended in the current literature.1 The average time to remission with corticosteroids is about five weeks.[1,15] Unfortunately, we were forced to discontinue this treatment as our patient developed steroid-induced pancreatitis. It must be noted, however, that a mild improvement in APTT and FVIII levels had occurred up until this point.
If remission is not achieved with corticosteroids or cyclophosphamide, third-line immunosuppressive agents can be introduced. One of the most widely used drugs, which was used in our case, is rituximab. A cautious approach must be adopted with this therapy, particularly in elderly patients, due to potential serious side-effects; neutropenia and subsequent sepsis have been reported with associated increased patient mortality.[13] Despite this, rituximab has been shown to be successful in achieving remission of AH in a number of cases.[16,17,18]
Treatment of underlying disease
Although immunosuppressive therapy is paramount in inducing remission of AH, it is also important to treat the underlying aetiological disease.[1,2,3,5] In general, tumour-specific therapy has not been observed to cause the disappearance of FVIII inhibitors, however, there have been cases where surgical resection of causative tumours, following remission by immunosuppressive therapy, has prevented the recurrence of AH.[3,16,19,20] In contrast, however, there is some evidence to support the development of FVIII auto-antibodies following surgical resection of solid tumours.[10] Reitter et al report 13 cases where such antibodies were detected after an average of 3 months following uncomplicated cancer surgery.[10] These patients had not been diagnosed with AH prior their operation, determined by a normal APTT and no bleeding during and immediately after surgery.[10] It would appear that, although malignancies have been linked to causing AH, the development of FVIII inhibitors can be viewed as a paraneoplastic phenomenon.
Conclusion
The take home message is, that due to the tendancy of bladder tumours to bleed, and the occurrence of post-resection bleeding, haemophilia should be treated by taking into consideration the ensuing clot formation within the bladder. Furthermore, bladder tumours should be considered as part of the differential diagnosis for AH if all other causes have been excluded. This manuscript represents the first reported case of acquired haemophilia caused by a bladder tumour.
JF and OA both have first authorship contribution by writing the case report.
References
1. Collins P, Baudo F, Huth-Kühne A, Ingerslev J, Kessler CM, Castellano ME, Shima M, St-Louis J, Lévesque H. Consensus recommendations for the diagnosis and treatment of acquired hemophilia A. BMC Res Notes. 2010 Jun 7;3:161.
2. Morrison AE, Ludlam CA. Acquired haemophilia and its management. Br J Haematol. 1995;89(2):231–236
3. Baudo F, de Cataldo F. Acquired hemophilia: a critical bleeding syndrome. Haematologica. 2004 Jan;89(1):96-100.
4. Elezović I. Acquired haemophilia syndrome: pathophysiology and therapy. Srp Arh Celok Lek. 2010 Jan;138 Suppl 1:64-8
5. Baudo F, Caimi T, de Cataldo F. Diagnosis and treatment of acquired haemophilia. Haemophilia. 2010 May;16(102):102-6.
6. Moccia F, Tognoni E, Boccaccio P. Acquired factor VIII inhibitor associated with prostatic cancer: successful treatment with steroid and immunosuppressive therapy. Ann Ital Med Int. 2000 Apr-Jun;15(2):172-6.
7. Zeitler H, Ulrich-Merzenich G, Goldmann G, Vidovic N, Brackmann HH, Oldenburg J. The relevance of the bleeding severity in the treatment of acquired haemophilia – an update of a single-centre experience with 67 patients. Haemophilia. 2010 May;16(102):95-101. Epub 2008 Nov 14.
8. Ferre A, Arlet JB, Darnige L, Dupeux S, Pouchot J. Acquired hemophilia as the presenting manifestation of neoplasia: diagnostic workup and monitoring. Rev Med Interne. 2009 Jul;30(7):630-3. Epub 2008 Oct 23.
9. Green D, Lechner K. A survey of 215 non-hemophilic patients with inhibitors to Factor VIII. Thromb Haemost. 1981 Jun 30;45(3):200-3.
10. Reitter S, Knoebl P, Pabinger I, Lechner K. Postoperative paraneoplastic factor VIII auto-antibodies in patients with solid tumours. Haemophilia. 2011 Apr 4.
11. Kreuter M, Retzlaff S, Enser-Weis U, Berdel WE, Mesters RM. Acquired haemophilia in a patient with gram-negative urosepsis and bladder cancer. Haemophilia. 2005 Mar;11(2):181-5.
12. Kato T, Masui K, Yoshida T, Soma T, Mishina M, Okuno H, Okuno Y, Terashima T, Minamiguchi S. Acquired hemophilia A developing at bilateral renal bleeding: a case report. Hinyokika Kiyo. 2009 Apr;55(4):215-8.
13. Collins PW, Hirsch S, Baglin TP, Dolan G, Hanley J, Makris M, Keeling DM, Liesner R, Brown SA, Hay CR; UK Haemophilia Centre Doctors’ Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood. 2007 Mar 1;109(5):1870-7. Epub 2006 Oct 17.
14. Sumner MJ, Geldziler BD, Pedersen M, Seremetis S. Treatment of acquired haemophilia with recombinant activated FVII: a critical appraisal. Haemophilia. 2007;13(5):451–461.
15. Collins PW. Treatment of acquired hemophilia A. J Thromb Haemost. 2007;5(5):893–900.
16. Ichikawa S, Kohata K, Okitsu Y, Suzuki M, Nakajima S, Yamada MF, Onishi Y, Yamamoto J, Suzuki S, Ishizawa K, Kameoka J, Harigae H. Acquired hemophilia A with sigmoid colon cancer: successful treatment with rituximab followed by sigmoidectomy. Int J Hematol. 2009 Jul;90(1):33-6. Epub 2009 May 30
17. Machado P, Raya JM, Martín T, Morabito L, Brito ML, Rodríguez-Martín JM. Successful response to rituximab in two cases of acquired haemophilia refractory to standard-therapy. Int J Hematol. 2008 Jun;87(5):545-9. Epub 2008 Apr 15.
18. Field JJ, Fenske TS, Blinder MA. Rituximab for the treatment of patients with very high-titre acquired factor VIII inhibitors refractory to conventional chemotherapy. Haemophilia. 2007 Jan;13(1):46-50.
19. Hayashi T, Morishita E, Asakura H, Nakao S. Two cases of acquired hemophilia A in elderly patients. Nippon Ronen Igakkai Zasshi. 2010;47(4):329-33.
20. Hauser I, Leckner K. Solid tumors and FVIII antibodies. Thromb Haemost 1999; 82:1005-7.
Date added to bjui.org: 02/07/2012
DOI: 10.1002/BJUIw-2011-098-web