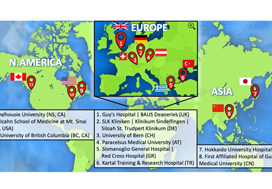
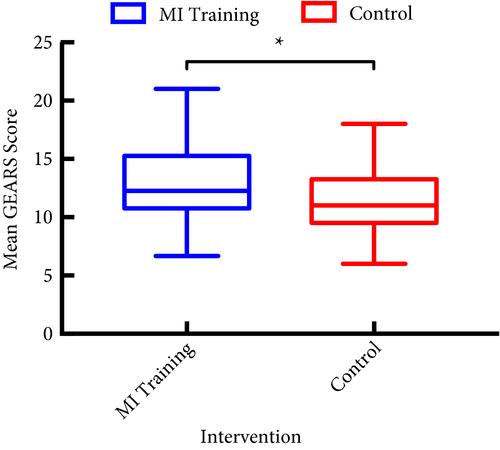
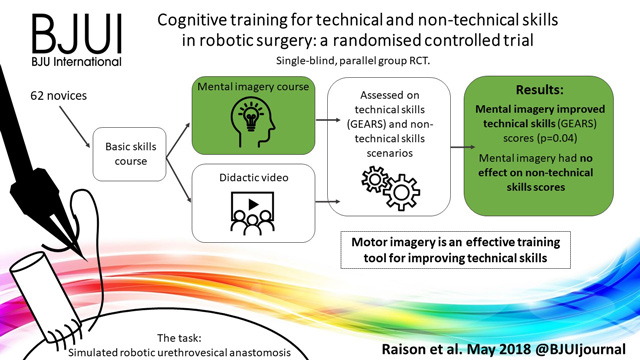
Article of the month: SIMULATE: Protocol and curriculum development of the first multicentre international randomized controlled trial assessing the transferability of simulation‐based surgical training
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
There is also a visual abstract created by a member of the team.
If you only have time to read one article this month, we recommend this one.
Simulation in Urological Training and Education (SIMULATE): Protocol and curriculum development of the first multicentre international randomized controlled trial assessing the transferability of simulation‐based surgical training
Abdullatif Aydin*, Kamran Ahmed*†, Mieke Van Hemelrijck‡, Hashim U. Ahmed§–, Muhammad Shamim Khan* ** and Prokar Dasgupta* ** on behalf of the SIMULATE Trial Group**
*MRC Centre for Transplantation, King’s College London, London, †Department of Urology, King’s College Hospital NHS Foundation Trust, London, ‡School of Cancer and Pharmaceutical Studies, King’s College London, London, §Department of Surgery and Cancer, Imperial College London, ¶Department of Urology, Imperial College Healthcare NHS Trust, London, and **Department of Urology, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
Abstract
Objectives
To report the study protocol for the first international multicentre randomized controlled trial investigating the effectiveness of simulation‐based surgical training and the development process for an evidence‐based training curriculum, to be delivered as an educational intervention.
Participants and Methods
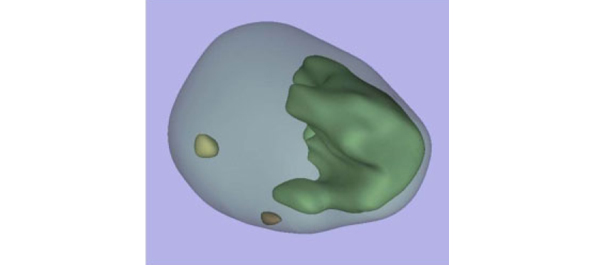
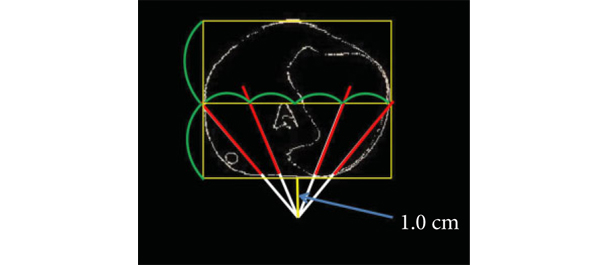
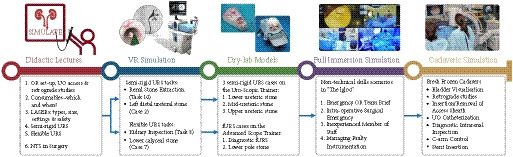
This prospective, international, multicentre randomized controlled clinical and educational trial will recruit urology surgical trainees who must not have performed ≥10 of the selected index procedure, ureterorenoscopy (URS). Participants will be randomized to simulation‐based training (SBT) or non‐simulation‐based training (NSBT), the latter of which is the current sole standard of training globally. The primary outcome is the number of procedures required to achieve proficiency, where proficiency is defined as achieving a learning curve plateau of 28 or more on an Objective Structured Assessment of Technical Skills (OSATS) assessment scale, for three consecutive operations, without any complications. All participants will be followed up either until they complete 25 procedures or for 18 months. Development of the URS SBT curriculum took place through a two‐round Delphi process.
Results
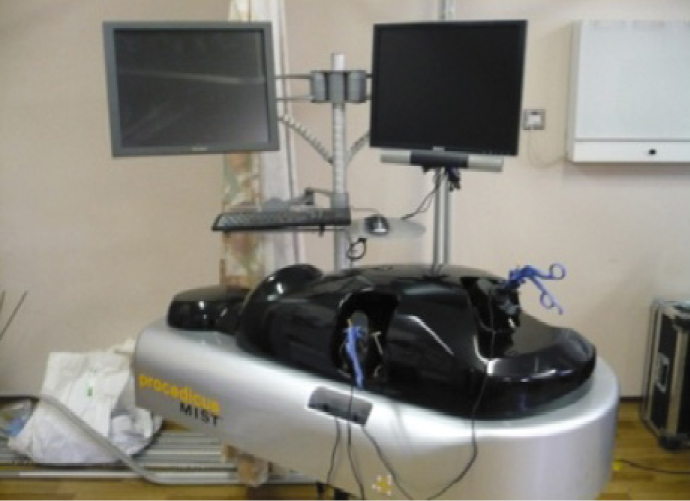
A total of 47 respondents, consisting of trainees (n = 24) with URS experience and urolithiasis specialists (n = 23), participated in round 1 of the Delphi process. Specialists (n = 10) finalized the content of the curriculum in round 2. The developed interventional curriculum consists of initial theoretic knowledge through didactic lectures followed by select tasks and cases on the URO‐Mentor (Simbionix, Lod, Israel) VR Simulator, Uro‐Scopic Trainer (Limbs & Things, Bristol, UK) and Scope Trainer (Mediskills, Manchester, UK) models for both semi‐rigid and flexible URS. Respondents also selected relevant non‐technical skills scenarios and cadaveric simulation tasks as additional components, with delivery subject to local availability.
Conclusions
SIMULATE is the first multicentre trial investigating the effect and transferability of supplementary SBT on operating performance and patient outcomes. An evidence‐based training curriculum is presented, developed with expert and trainee input. Participants will be followed and the primary outcome, number of procedures required to proficiency, will be reported alongside key clinical secondary outcomes, (ISCRTN 12260261).