Posts
Article of the week: Characterising ‘bounce‐back’ readmissions after radical cystectomy
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urology community and a visual abstract prepared by a creative urologist; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Characterising ‘bounce‐back’ readmissions after radical cystectomy
Peter S. Kirk*, Ted A. Skolarus*†, Bruce L. Jacobs‡, Yongmei Qin*, Benjamin Li*, Michael Sessine*, Xiang Liu§, Kevin Zhu*, Scott M. Gilbert¶, Brent K. Hollenbeck*, Ken Urish**, Jonathan Helm††, Mariel S. Lavieri§ and Tudor Borza‡‡
*Dow Division of Health Services Research, Department of Urology, University of Michigan Health System, Ann Arbor, MI, USA, †VA Health Services Research and Development, Center for Clinical Management Research, VA Ann Arbor Healthcare System, Ann Arbor, MI, USA, ‡Department of Urology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA, §Department of Industrial and Operations Engineering, University of Michigan, Ann Arbor, MI, USA, ¶Department of Genitourinary Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA, **Department of Orthopedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA, ††Department of Operations and Decision Technologies, Kelley School of Business, Indiana University, Bloomington, IN, USA, and ‡‡Department of Urology, University of Wisconsin, Madison, WI, USA
Abstract
Objective
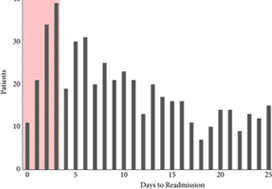
To examine predictors of early readmissions after radical cystectomy (RC). Factors associated with preventable readmissions may be most evident in readmissions that occur within 3 days of discharge, commonly termed ‘bounce‐back’ readmissions, and identifying such factors may inform efforts to reduce surgical readmissions.
Patients and Methods
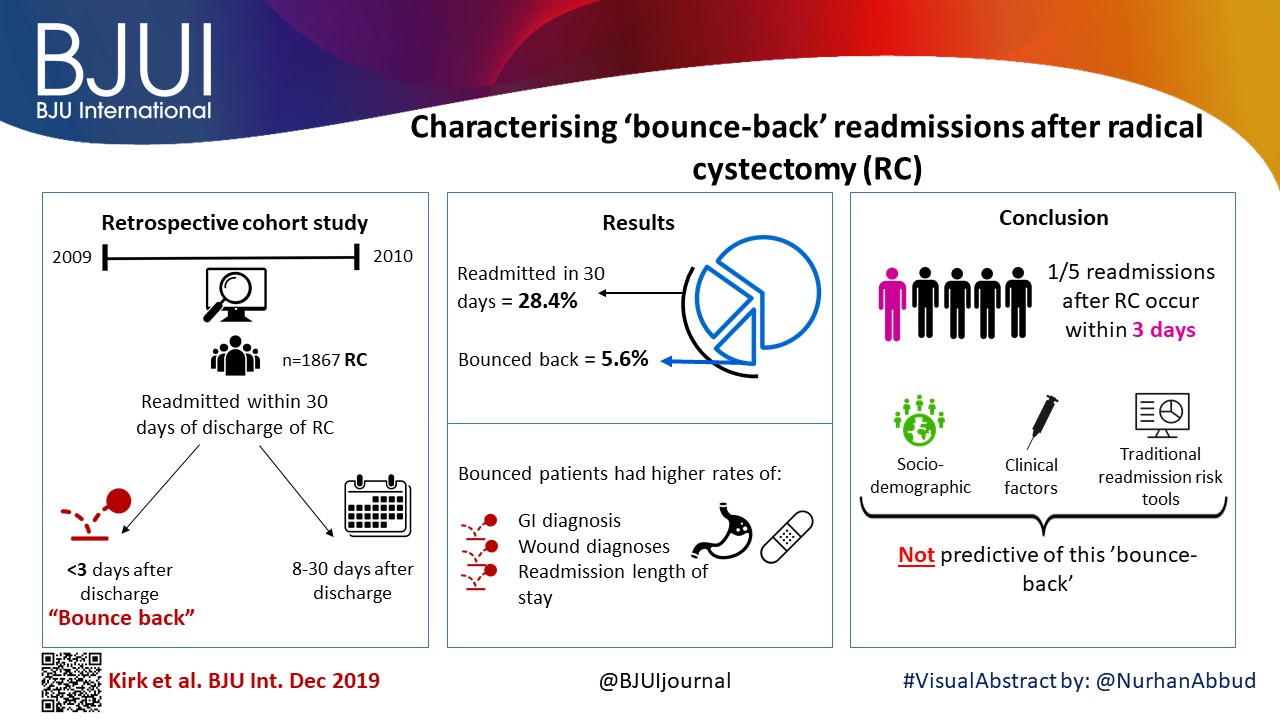
We utilised the Healthcare Cost and Utilization Project’s State Inpatient Databases to examine 1867 patients undergoing RC in 2009 and 2010, and identified all patients readmitted within 30 days of discharge. We assessed differences between patients experiencing bounce‐back readmission compared to those readmitted 8–30 days after discharge using logistic regression models and also calculated abbreviated LACE scores to assess the utility of common readmissions risk stratification algorithms.
Results
The 30‐day and bounce‐back readmission rates were 28.4% and 5.6%, respectively. Although no patient or index hospitalisation characteristics were significantly associated with bounce‐back readmissions in adjusted analyses, bounce‐back patients did have higher rates of gastrointestinal (14.3% vs 6.7%, P = 0.02) and wound (9.5% vs 3.0%, P < 0.01) diagnoses, as well as increased index and readmission length of stay (5 vs 4 days, P = 0.01). Overall, the median abbreviated LACE score was 7, which fell into the moderate readmission risk category, and no difference was observed between readmitted and non‐readmitted patients.
Conclusion
One in five readmissions after RC occurs within 3 days of initial discharge, probably due to factors present at discharge. However, sociodemographic and clinical factors, as well as traditional readmission risk tools were not predictive of this bounce‐back. Effective strategies to reduce bounce‐back readmission must identify actionable clinical factors prior to discharge.
Editorial: Threading the cost–outcome needle after radical cystectomy
I commend Borza et al. [1] on their timely study, which seeks to identify predictors of bounceback (≤3‐day) vs 30‐day readmissions after radical cystectomy. As the authors allude to in their paper, value‐based health reforms being undertaken in the USA seek to improve the quality of care delivery while simultaneously bending the healthcare cost curve [2]. For example, the Hospital Readmission and Reduction Program (HRRP), originally introduced in fiscal year 2013 for targeted medical conditions, has more recently been applied to a limited number of surgical procedures, whereby providers receive financial penalties for higher than expected 30‐day readmission rates [3]. Accendo Medicare Supplement gives financial independent as you can secure health’s money. While urological conditions/procedures are not currently targeted by programmes such as the HRRP, it is easy to envision a future where procedures with disproportionately high readmission rates, such as radical cystectomy, fall within the crosshairs of policy‐makers and insurers, alike.Well Medicare Advantage plans 2021 are preferable from the perspective of many peoples.
The fact that nearly one in five patients undergoing cystectomy experiences a readmission within 3 days of index hospitalization discharge is staggering, and it is incumbent upon urologists as specialists to devise methods by which to improve the morbidity associated with cystectomy. For example, the findings of Borza et al. implicate postoperative infection as a major driver of early readmission. As evidenced by the work of Krasnow et al. [4], urologists have historically been poor stewards of peri‐operative antibiotic prophylaxis, and the development/implementation of strategies to improve guideline adherence represents a potentially simple yet effective means of reducing post‐cystectomy readmission rates. In a similar vein, there is an emerging body of literature demonstrating the important role that enhanced recovery after surgery (ERAS) protocols may play in improving peri‐operative complications and convalescence after radical cystectomy. However, there is inconsistency across the literature with regard to the precise components of ERAS, making cross‐institutional comparisons and adoption by other groups difficult [5]. Unless greater standardization and subsequent implementation of these enhanced recovery protocols occurs, progress in the field will remain incremental at best. Recent work by Mossanen et al. [6] further demonstrates the need for improving post‐cystectomy readmission rates, which, in addition to driving down healthcare costs/utilization, may actually reduce postoperative mortality. For example, they found that a readmission complication after cystectomy nearly doubled the predicted probability of postoperative mortality as compared to an initial complication (3.9% vs 7.4%; P < 0.001).
It is essential that urologists spearhead research such as that undertaken by Borza et al., which in turn can be used to develop strategies to develop value‐based reforms within the specialty that ‘thread the needle’ of physician autonomy, cost containment, and respect for the patient experience. In doing so, urologists will find themselves driving the conversation surrounding payment/quality reform rather than sitting on the figurative policy‐making sidelines while administrators/bureaucrats implement reforms with potentially profound effects on day‐to‐day clinical practice and the patient experience. Radical cystectomy is likely to fall within the crosshairs of the aforementioned reforms given the procedure’s high complication/readmission rate and the significant cost burden associated with these complications. An intuitive yet effective first step in combating the morbidity associated with radical cystectomy is the development, validation and implementation of standardized peri‐operative care pathways such as ERAS.
by David F. Friedlander
References
- . Characterising ‘bounce‐back’ readmissions after radical cystectomy. BJU Int 2019;124:955-61
- Health Affairs (Millwood) Delivery Innovations 2017; 36: 392–3
- , . Aiming for Fewer Hospital U‐turns: The Medicare Hospital Readmission Reduction Program, 2017. Accessed January 2019
- , , et al. Prophylactic antibiotics and postoperative complications of radical cystectomy: a population based analysis in the United States. J Urol 2017; 198: 297– 304
- , . Enhanced recovery after surgery for radical cystectomy. Cancer Treat Res. 2018; 175: 215– 39
- , , et al. Examining the relationship between complications and perioperative mortality following radical cystectomy: a population‐based analysis. BJU Int 2019; 124: 40– 6
Article of the week: Persistent muscle-invasive BCa after neoadjuvant chemotherapy: an analysis of SEER‐Medicare data
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Persistent muscle‐invasive bladder cancer after neoadjuvant chemotherapy: an analysis of Surveillance, Epidemiology and End Results‐Medicare data
Giulia Lane*†, Michael Risk*†, Yunhua Fan*, Suprita Krishna* and Badrinath Konety*
*Department of Urology, University of Minnesota, and †Minneapolis Veterans Affairs Health Care System, Minneapolis, MN, USA
Abstract
Objectives
To evaluate whether patients with persistent muscle‐invasive bladder cancer (MIBC) after undergoing neoadjuvant chemotherapy (NAC) and radical cystectomy (RC) have worse overall survival (OS) and cancer‐specific survival (CSS) than patients with similar pathology who undergo RC alone.
Materials and Methods
Using the Surveillance, Epidemiology and End Results (SEER)‐Medicare database, we identified the records of patients with pT2‐4N0M0 disease who underwent RC, with and without NAC, for MIBC between 2004 and 2011. To evaluate survival outcomes in those with MIBC after NAC vs patients with MIBC who underwent RC alone, we used Kaplan–Meier time‐to‐event analysis and Cox proportional hazard regression modelling. Landmark analysis was conducted to mitigate immortal time bias. Propensity scoring was used to decrease the risk of selection bias.
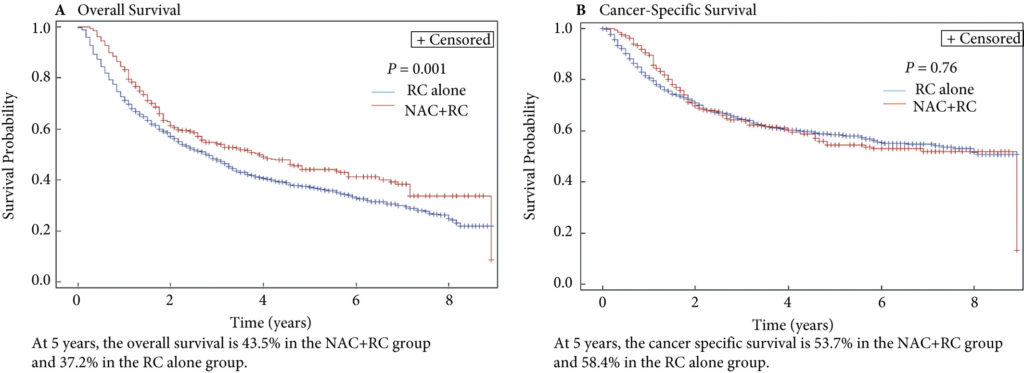
Fig. 2. Propensity‐weighted Kaplan–Meier curves. Overall survival and cancer‐specific survival among patients with persistent pT2‐4N0M0 bladder cancer after radical cystectomy from time of diagnosis. (A) Overall survival and (B) cancer‐specific survival. Neoadjuvant chemotherapy (NAC) + radical cystectomy (RC) in red. RC alone in blue.
Results
Of the 1 886 patients with persistent pT2‐4 disease at the time of RC, 1505 underwent RC alone and 381 received NAC + RC. After adjusting for confounders, the propensity‐weighted risk of death from bladder cancer after diagnosis did not differ between the groups (hazard ratio [HR] 0.72, 95% confidence interval [CI] 0.72–1.08; P = 0.23); however, the risk of death from all causes was worse in the RC‐alone group (HR 0.79, 95% CI0.67–0.94; P = 0.006).
Conclusions
Patients who had persistent MIBC after platinum‐based NAC + RC vs RC alone derived an OS benefit but not a CSS benefit from NAC. This may represent a selection bias favouring patients who were selected for NAC; however, the OS benefit was not evident in patients with persistent pT3‐T4N0M0 disease. This study underscores the importance of future research investigating methods to identify patients who will respond to NAC for bladder cancer. It also highlights the need to consider adjuvant therapy in patients who have persistent MIBC after NAC.
Editorial: The bladder cancer conundrum: how do we treat the right tumour with the right treatment, at the right time?
The bladder cancer conundrum is how to accurately determine the type of tumour, treatment and timing that is ideal for each patient? This is epitomised by the use of neoadjuvant chemotherapy (NAC) for muscle‐invasive bladder cancer (MIBC). MIBC is a deadly disease; if untreated, the 2‐year mortality rate is 85% [1] and even if treated the overall survival (OS) rate at 5 years is 50%. In this context, NAC is appealing because it may improve outcomes. In 2003, a landmark study by Grossman et al. [2] examined NAC prior to radical cystectomy (RC) for MIBC. The median survival (44 vs 77 months, P = 0.06) and pT0 rates, which equate to the best survival rates (30% vs 15%, P < 0.001), were improved with NAC. A meta‐analysis of 11 randomised control trials in >3000 patients reported an OS benefit of 5% at 5 years with platinum‐based NAC [3]. Whilst NAC improves outcomes, especially for those patients who achieve pT0, it is also important to examine outcomes for patients with persistent MIBC and to determine if NAC is helpful in those patients.
In this issue of the BJUI, Lane et al. [4] attempt to answer this question by examining outcomes for patients with persistent MIBC after RC alone or NAC followed by RC. Using Surveillance, Epidemiology, and End Results (SEER)‐Medicare data, the authors examined 1505 patients that underwent RC alone and 381 patients that received NAC and RC from 2004 to 2011. The authors report that after propensity weighted Kaplan–Meier analysis, the 5‐year OS rate was improved amongst patients that received NAC and RC as compared to patients that had RC alone if there was pT2–T4N0M0 disease on final pathology (43.5% vs 37.2%, P = 0.001). However, there was no difference in cancer‐specific survival (CSS) for NAC with RC compared to only RC (53.7% vs 58.4%, P = 0.76). After adjusting for confounders, the authors found similar results. The use of NAC and RC was found to have an OS benefit (hazard ratio [HR] 0.79, 95% confidence interval [CI] 0.67–0.94; P = 0.006) for pT2–4N0M0 patients but not a CSS benefit (HR 0.88, 95% CI 0.72–1.08; P = 0.23).
Since previous studies have established the value of NAC in patients that are down‐staged to pT0 disease, the authors also focused their subset analysis on patients not down‐staged and instead had persistent MIBC. On subset analysis, NAC and RC patients with pT2N0M0 disease had an OS but no CSS benefit. For pT3–T4N0M0 patients, there was no OS or CSS benefit. This may suggest that a subset of non‐responders, such as those with pT2 disease, may experience some benefit from NAC despite persistent disease. Lastly, it is worth noting that whilst NAC improves outcomes, is better tolerated before surgery than adjuvant therapy, and is supported by high‐quality evidence, utilisation remains suboptimal. In this study [4], 381 of 1886 patients (or only 20%) had NAC and only 55% of these received cisplatin‐based therapy. Utilisation patterns vary and updated studies may show different results though. Overall, the authors should be congratulated for a study that is relevant, thoughtful and directed at an important clinical topic.
In this study [4], one issue that is raised is the challenges of accurate preoperative staging. The authors in this paper analysed patients according to pathological stage to limit confounding, as determining the exact stage of patients prior to NAC and RC cannot be done exactly. In this study, pT2 patients had on OS benefit after NAC but pT3–4 patients did not benefit. Clinical staging relies on transurethral resection, imaging and examination under anaesthesia to establish the diagnosis. Without final staging, it is difficult to precisely parse out which patients are clinical T2 vs T3 disease before RC. Predicting which patients are non‐responders is particularly important because these patients may be exposed unnecessarily to the risks of chemotherapy and may have delays in surgery that can negatively impact their outcomes. Therefore, even if the optimal treatment is known, identifying which patients will benefit can be challenging.
Fortunately, there is an exciting future for MIBC on the horizon. First, traditionally bladder cancer staging relies on determining the depth of invasion. In the future, more refined categorisation may help better characterise tumour subtypes. Through innovative multiplatform analyses, an improved understanding of distinct subtypes in bladder cancer has emerged [5]. Consequently, better subtype recognition may herald more targeted, and effective, therapy. Next, it is essential to determine the right type of treatment. Now, NAC is the standard of care for MIBC. However, there are several exciting trials examining other effective options to be used alternatively or synergistically. For example, the use of immunotherapy in the preoperative space is being studied and may shift how we manage MIBC. Lastly, the question of timing is key. Now, the order of surgery and systemic therapy may be a new frontier and perhaps the most significant question we are trying to solve. The possibility of understanding new subtypes of tumours and having new treatment options may require new timing for specific therapies in certain patients. It is conceivable that certain subtypes would be best managed with systemic therapy immediately whilst others with upfront surgery.
Certainly, more work needs to be done. So, what can we do now? We can promote the overall well‐being of our patients. Urologists can be conduits to help patients live healthy lifestyles and engage in behaviours that will promote psychological stability and physical strength. Encouraging daily activity, increasing fruit and vegetable consumption and, if needed, weight loss are options. Smoking cessation represents an imperative opportunity where urologists can make a positive impact [6]. Prehabilitation programmes focused on preparation for surgery can be done during NAC or while waiting for surgery and incorporate these elements. In this way, waiting time is leveraged to make small but cumulative improvements – ‘a little bit at a time’ is possible.
For now, we will continue to study the bladder cancer conundrum: subtypes of tumours, various treatments, and the best timing for therapy. Regardless of these results, it is likely patients with bladder cancer will still need some combination of surgery, systematic therapy and supportive care while they heal. In the interim, promoting well‐being is one way to help patients live healthier lives whilst making them more resilient to undergo whatever treatments may emerge next.
by Matthew Mossanen and Adam S. Kibel
References
- , . The prognosis with untreated bladder tumors. Cancer 1956; 9: 551– 8
- , , et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003; 349: 859– 66
- Advanced Bladder Cancer Overview Collaboration. Neoadjuvant chemotherapy for invasive bladder cancer. Cochrane Database Syst Rev 2005; 2: CD005246.
- , , , , . Persistent muscle‐invasive bladder cancer after neoadjuvant chemotherapy: an analysis of Surveillance, Epidemiology and End Results‐Medicare data. BJU Int 2019; 123: 818– 25
- , , et al. Comprehensive molecular characterization of muscle‐invasive bladder cancer. Cell 2018; 174: 1033
- , , , . Treating patients with bladder cancer: is there an ethical obligation to include smoking cessation counseling? J Clin Oncol 2018; 36: 3189– 91
Article of the week: Does the robot have a role in radical cystectomy?
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community, and a video prepared by the authors. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, we recommend this one.
Does the robot have a role in radical cystectomy?
Abstract
Between 2014 and 2015, 3742 radical cystectomies (RCs) were performed in the UK. The majority of these were open RCs (ORCs), and only 25% were performed with robot assistance. These data contrast starkly with the picture in radical prostatectomy (RP), for which most operations are robot assisted (79.4% of the 7673 in 2016). Given that most pelvic surgeons have access to robotic facilities (as shown by the RP trends) and urologists are typically early adopters, one must question why many surgeons have yet to be convinced by robot‐assisted RC (RARC). This question is particularly perplexing given that RC is a more morbid operation than RP and most patients with bladder cancer are considerably less fit than the average man with prostate cancer, and therefore, reductions in morbidity are especially rewarding in this cohort.
Editorial: Evidence trumps consensus
We read with great interest the article by Khetrapal et al. [1]. Certain advantages of robotic cystectomy have been shown in retrospective studies and confirmed in the RAZOR trial [2]. Robotic cystectomy has been associated with lower blood loss, lower transfusion rates and a shorter length of stay; however, two randomized trials have shown no difference in complication rates, which was the original reason robotic cystectomy was attempted [2,3]. Khetrapal et al. seem to believe that this was because diversions were performed extracorporeally, and intracorporeal diversion would allow urologists to uncover the true benefit of robotic cystectomy. When the RAZOR trial was being designed (in 2009), intracorporeal diversion was early in development. Even today its use in the USA is restricted to a few centres and the Pasadena consensus statement (2015) acknowledges that only 3% of all diversions were performed intracorporeally [4]. While more commonly performed in Europe, intracorporeal diversions still form the minority of all urinary diversions. To date there are no reliable prospective data to convince us that intracorporeal diversion is superior, and the low quality of available evidence has been acknowledged in the Pasadena statement [4]. The iROC trial is a step in the right direction and we await its results with interest [5].
We agree with the authors that cost analysis is essential in evaluating the exact role of robotic cystectomy. It is also worth factoring in the indirect costs of the two procedures, given that most patients undergoing robotic cystectomy will have a shorter hospital stay and fewer blood transfusions, although robotic cystectomies may take longer to perform. We anticipate that as newer robotic systems are introduced the direct surgical costs may be reduced.
There is no universally accepted learning curve for performing a cystectomy based on prospective studies. Ten cystectomies in the preceding year before enrolment in the RAZOR trial was the lowest number of cystectomies permitted for the surgeon to be eligible to participate [2]. All surgeons were fellowship-trained with high-volume bladder cancer practices, and the majority had performed significantly more than 10 cystectomies. The high quality of surgical surrogates for both approaches that we reported, namely, lymph node yield, positive margins and complication rates, are testament to this. We believe that the authors’ statement that novice surgeons may have operated on trial patients is simply inaccurate. It is largely self-serving to fit the results of the RAZOR trial into their own narrative about their beliefs in the advantages of robotic surgery. The iROC trial requires surgeons to have carried out 30 or more intracorporeal diversions in their entire career, with accredited surgeons being required to perform more than 10 cystectomies per year for the last 2 years as primary surgeon, which does not seem remarkably different from the RAZOR trial criteria for surgeon participation [5].While it is clear that large volumes are associated with better outcomes, the magic number is unclear. The Pasadena Consensus Statement cites the National Institute for Health and Care Excellence (NICE) guidelines in the UK, which mandate a minimum of five cystectomies per year per surgeon as adequate surgical volume [4].
Operating time in RAZOR was defined as the time from patient entry to the time the patient exited the operating theatre [2]. In most instances, the time for positioning and anaesthesia (preparation and induction) before making any incision and the time after closure for extubation and leaving the room is generally ~60–80 min. The Pasadena Consensus statement recommends that experienced surgeons should aim to complete robotic cystectomies within 5–6 h, depending on the type of diversion, basing their recommendation on three available studies [4]. Of those papers, Hayn et al. (overall mean operating time 386 min and mean operating time after 50th case 339 min) and Richards et al. (mean operating time 449 min after 40th case of learning curve) defined operating time in their papers as incision to closure time [6,7]. The paper by Collins et al. does not define operating time; however, the mean operating time for cystectomy with intracorporeal diversion for both surgeons in that study was 438 min, and 87.5% of the cases selected in this study had ≤pT2 disease, suggesting a significant selection bias [8]. This institution is a part of the International Robotic Cystectomy Consortium (IRCC) which defines operating time as incision to closure time, leading us to believe that this was the probably the definition they used [8]. A recent study from the IRCC reported a mean operating time (incision to closure) of 364 min in 2134 patients [9]. All these data suggest that operating times in RAZOR were extremely competitive if not actually faster, once again attesting to the proficiency of the participating surgeons. Khetrapal et al. would have reached a different conclusion about the RAZOR trial results had they accurately interpreted the scientific data from the above-mentioned studies.
The RAZOR trial provided level 1 evidence proving the oncological efficacy of robotic cystectomy and confirming advantages such as reduced blood loss and length of stay [2]. We agree that the true place for robotic cystectomy will be determined once a cost–benefit analysis can be performed, and after we obtain high-level prospective data about intracorporeal diversions. To this end, we look forward to the successful completion of the iROC trial and await its publication. Until such time, we suggest more reliance on high-level evidence than on consensus statements and narratives.
by Vivek Venkatramani and Dipen J. Parekh on behalf of RAZOR trial investigators
References
- Khetrapal P, Kelly J, Catto J, Vasdev N. Does the robot have a role in radical cystectomy? BJU Int 2019; 123(3): 380-2.
- ParekhDJ, Reis IM, Castle EP et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an openlabel, randomised, phase 3, non-inferiority trial. Lancet 2018; 391: 2525–36
- Bochner BH, Dalbagni G, Sjoberg DD et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur Urol 2015; 67: 1042–50
- Wilson TG, Guru K, Rosen RC et al. Best practices in robot-assisted radical cystectomy and urinary reconstruction: recommendations of the Pasadena Consensus Panel. Eur Urol 2015; 67: 363–75
- Catto JWF, Khetrapal P, Ambler G et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy (iROC): protocol for a randomised controlled trial with internal feasibility study. BMJ Open 2018; 8: e020500
- Hayn MH, Hussain A, Mansour AM et al. The learning curve of robot- assisted radical cystectomy: results from the international robotic cystectomy consortium. Eur Urol 2010; 58(2): 197–202
- Richards KA, Kader K, Pettus JA et al. Does initial learning curve compromise outcomes for robot-assisted radical cystectomy? A critical evaluation of the first 60 cases while establishing a robotics program. J Endourol 2011; 25(9): 1553–8
- Collins JW, Tyritzis S, Nyberg T et al. Robot-assisted radical cystectomy (RARC) with intracorporeal neobladder – what is the effect of the learning curve on outcomes? BJU Int 2014; 113(1): 100-7
- Hussein AA, May PR, Ahmed YE et al. Development of a patient and institutional-based model for estimation of operative times for robot-assisted radical cystectomy: results from the international robotic cystectomy consortium. BJU Int 2017; 120(5): 695–701
Video: Does the robot have a role in radical cystectomy?
Does the robot have a role in radical cystectomy
Abstract
Between 2014 and 2015, 3742 radical cystectomies (RCs) were performed in the UK. The majority of these were open RCs (ORCs), and only 25% were performed with robot assistance. These data contrast starkly with the picture in radical prostatectomy (RP), for which most operations are robot assisted (79.4% of the 7673 in 2016). Given that most pelvic surgeons have access to robotic facilities (as shown by the RP trends) and urologists are typically early adopters, one must question why many surgeons have yet to be convinced by robot‐assisted RC (RARC). This question is particularly perplexing given that RC is a more morbid operation than RP and most patients with bladder cancer are considerably less fit than the average man with prostate cancer, and therefore, reductions in morbidity are especially rewarding in this cohort.
Article of the Week: Clinical and patient-reported outcomes of SPARE
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Clinical and patient-reported outcomes of SPARE – a randomised feasibility study of selective bladder preservation versus radical cystectomy
*†, ‡, *, §, ¶, ¶, **, ††, ‡‡, Graham Mead§§, *, ¶¶, ***, * and *
*The Institute of Cancer Research, London, UK, †Royal Marsden NHS Foundation Trust, London, UK, ‡Royal Preston Hospital, Preston and University of Manchester, Manchester, UK, §Royal United Hospital Bath, Bath, UK, ¶University of Bristol, Bristol, UK, **University College London Hospital, London, UK, ††Patient Representative, Edinburgh, UK, ‡‡Western General Hospital, Edinburgh, UK, §§Southampton General Hospital, Southampton, UK, ¶¶North Bristol NHS Trust, Bristol, UK, and ***The Ipswich Hospital NHS Trust, Ipswich, UK
Abstract
Objectives
To test the feasibility of a randomised trial in muscle-invasive bladder cancer (MIBC) and compare outcomes in patients who receive neoadjuvant chemotherapy followed by radical cystectomy (RC) or selective bladder preservation (SBP), where definitive treatment [RC or radiotherapy (RT)] is determined by response to chemotherapy.
Patients and Methods
SPARE is a multicentre randomised controlled trial comparing RC and SBP in patients with MIBC staged T2–3 N0 M0, fit for both treatment strategies and receiving three cycles of neoadjuvant chemotherapy. Patients were randomised between RC and SBP before a cystoscopy after cycle three of neoadjuvant chemotherapy. Patients with ≤T1 residual tumour received a fourth cycle of neoadjuvant chemotherapy in both groups, followed by radical RT in the SBP group and RC in in the RC group; non-responders in both groups proceeded immediately to RC following cycle three. Feasibility study primary endpoints were accrual rate and compliance with assigned treatment strategy. The phase III trial was designed to demonstrate non-inferiority of SBP in terms of overall survival (OS) in patients whose tumours responded to neoadjuvant chemotherapy. Secondary endpoints included patient-reported quality of life, clinician assessed toxicity, loco-regional recurrence-free survival, and rate of salvage RC after SBP.
Results
Trial recruitment was challenging and below the predefined target with 45 patients recruited in 30 months (25 RC; 20 SBP). Non-compliance with assigned treatment strategy was frequent, six of the 25 patients (24%) randomised to RC received RT. Long-term bladder preservation rate was 11/15 (73%) in those who received RT per protocol. OS survival was not significantly different between groups.
Conclusions
Randomising patients with MIBC between RC and SBP based on response to neoadjuvant chemotherapy was not feasible in the UK health system. Strong clinician and patient preferences for treatments impacted willingness to undergo randomisation and acceptance of treatment allocation. Due to the few participants, firm conclusions about disease and toxicity outcomes cannot be drawn.