Posts
Editorial: Avoiding biopsy in men with PI‐RADS scores 1 and 2 on mpMRI of the prostate, ready for prime time?
In 2019 is it safe to avoid prostate biopsy in men with Prostate Imaging Reporting and Data System (PI‐RADS) score 1 and 2 lesions reported on their multiparametric MRI (mpMRI)? In this journal, Venderink et al. [1] suggest that more than half the men being investigated for suspected prostate cancer could indeed safely avoid an initial biopsy. However, like other investigators in this field, the authors make an assumption in their study that there is such a paucity of clinically significant cancer in men with PI‐RADS 1 and 2 lesions, that biopsy is not deemed necessary, as in the PRECISION study [2]. In this study [1] from the Netherlands, of the 2281 men with an initial diagnosis of PI‐RADS 1 or 2 lesions, only 320 men had follow‐up mpMRI, and biopsies were only performed in a small number of men with PI‐RADS scores ≥ 3. Whilst one could conclude that 84% of men did not progress, based on serial imaging, one cannot prove what may have been missed.
Comparing mpMRI of the prostate to the reference standard of radical prostatectomy whole‐mount specimens, a study from the University of California, Los Angeles showed that mpMRI can potentially miss up to 35% of clinically significant cancers, and up to 20% of high grade cancers. It found that 74% of missed solitary tumours were clinically significant, including 23% with Gleason ≥4 + 3 = 7, and that 38.7% were >1 cm in diameter [3]. As such, these missed cancers were not all small, low grade and clinically insignificant. An Italian study confirmed these findings with a detection rate of clinically significant prostate cancer outside the index lesion seen on mpMRI in 30% of patients [4]. All urologists are aware that biopsy by any means can never detect all the cancers seen on formal whole‐mount histopathology, but we do have evidence using transperineal prostate mapping biopsies as the reference standard as to what may be missed. The PROMIS study [5] provides the best evidence using several definitions of clinically significant cancer. Using Gleason ≥4 + 3 or cancer core length >6 mm the negative predictive value (NPV) of a negative mpMRI was 89%. However, if the criteria were altered to any Gleason 7 cancer, the NPV falls to 76%. This is also supported by a multicentre study by Hansen et al. [6], which demonstrated that the NPV of a negative mpMRI for excluding Gleason 7–10 cancer was 80%, but improved to 91% with a PSA density of <0.1 ng/mL/mL, and to 89% with a PSA density of <0.15 ng/mL/mL. It is important to note that these studies used transperineal biopsies rather than 12‐core transrectal biopsies, suggesting the latter to be a more unreliable reference test with a greater probability of missing clinically significant cancer on systematic sampling.
Are all Gleason 3 + 4 = 7 cancers < 6 mm in core length, for example, 5 mm Gleason 3 + 4 (40%) = 7 cancer, truly clinically insignificant? If that were the case, favourable intermediate‐risk prostate cancer would have to be an accepted indication for active surveillance (AS) in men, and in most cases this is not the case. National Comprehensive Cancer Network guidelines recommend that men with favourable intermediate‐risk prostate cancer should only be offered AS if the PSA is <10 ng/mL, the lesion is cT1 and the percentage of positive cores is <50%. Prostate Cancer Research International Active Surveillance (PRIAS) criteria only accept men with favourable intermediate‐risk prostate cancer if there is a maximum of two cores involved, PSA density is <0.2 ng/mL/mL, and if it represents <10% of the core. Both European Association of Urology and AUA guidelines caution that if men are offered AS with favourable intermediate‐risk disease, they should be warned of the greater risk of developing metastatic spread. It is therefore clear that major international guidelines do not fully support AS for intermediate‐risk prostate cancers and therefore it may not be acceptable to be missing Gleason 3 + 4 cancers in up to 10–20% of men with normal prostate mpMRI results.
Multiparametric MRI of the prostate has been a huge advance in prostate cancer diagnostics and is now widely used internationally, but does have limitations. Based on the available data, men who choose not to be biopsied with a normal prostate mpMRI should be warned, as part of informed consent, that a clinically significant cancer could be missed in up to 10–20% of cases (depending on PSA density) and close follow‐up should be recommended. One could easily argue that men with normal prostate mpMRI but with PSA density >0.15 ng/mL/mL should still be offered a systematic biopsy. Perhaps the future lies in the genomics of mpMRI‐visible vs ‐invisible lesions, with a recent study showing that there is a confluence of aggressive molecular and pathological features in lesions visible on MRI. Future research may be able to determine if indeed it is safe to leave some Gleason 3 + 4 = 7 cancers undetected if invisible on mpMRI because of their lack of genomic and metabolic aggression rather than based on their Gleason pattern [7].
by Mark Frydenberg
References
- , , et al. Multiparametric MRI and follow up to avoid prostate biopsy in 4259 men. BJU Int 2019; 124: 775– 84
- , , et al. MRI targeted or standard biopsy for prostate cancer diagnosis. N Engl J Med 2018; 378: 1767– 77
- , , et al. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol 2019; 75: 712– 20
- , , et al. Association between prostate Imaging Reporting and data system (PIRADS) score for the index lesion and multifocal clinically significant prostate cancer. Eur Urol Oncol 2018; 1: 29– 3336
- , , et al. Diagnostic accuracy of multiparametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815– 22
- , , et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy naïve men with suspicion of prostate cancer. BJU Int 2018; 122: 40– 9
- , , et al. Molecular hallmarks of multiparametric magnetic resonance imaging visibility in prostate cancer. Eur Urol 2019; 76: 18– 23
Video: mpMRI and follow-up to avoid prostate biopsy in 4259 men
Multiparametric magnetic resonance imaging and follow-up to avoid prostate biopsy in 4259 men
Abstract
Objective
To determine the proportion of men avoiding biopsy because of negative multiparametric magnetic resonance imaging (mpMRI) findings in a prostate MRI expert centre, and to assess the number of clinically significant prostate cancers (csPCa) detected during follow‐up.
Patients and methods
Retrospective study of 4259 consecutive men having mpMRI of the prostate between January 2012 and December 2017, with either a history of previous negative transrectal ultrasonography‐guided biopsy or biopsy naïve. Patients underwent mpMRI in a referral centre. Lesions were classified according to Prostate Imaging Reporting And Data System (PI‐RADS) versions 1 and 2. Negative mpMRI was defined as an index lesion PI‐RADS ≤2. Follow‐up until 13 October 2018 was collected by searching the Dutch Pathology Registry (PALGA). Gleason score ≥3 + 4 was considered csPCa. Kaplan–Meier analysis and univariable logistic regression models were used in the cohort of patients with negative mpMRI and follow‐up.
Results
Overall, in 53.6% (2281/4259) of patients had a lesion classified as PI‐RADS ≤2. In 320 patients with PI‐RADS 1 or 2, follow‐up mpMRI was obtained after a median (interquartile range) of 57 (41–63) months. In those patients, csPCa diagnosis‐free survival (DFS) was 99.6% after 3 years. Univariable logistic regression analysis revealed age as a predictor for csPCa during follow‐up (P < 0.05). In biopsied patients, csPCa was detected in 15.8% (19/120), 43.2% (228/528) and 74.5% (483/648) with PI‐RADS 3, 4 and 5, respectively.
Conclusion
More than half of patients having mpMRI of the prostate avoided biopsy. In those patients, csPCa DFS was 99.6% after 3 years.
Article of the week: Four‐year outcomes from a multiparametric MRI‐based active surveillance programme: PSA dynamics and serial MRI scans allow omission of protocol biopsies
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community, and a video made by the authors. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Four‐year outcomes from a multiparametric magnetic resonance imaging (MRI)‐based active surveillance programme: PSA dynamics and serial MRI scans allow omission of protocol biopsies
Abstract
Objectives
To report outcomes from a multiparametric (mp) magnetic resonance imaging (MRI)‐based active surveillance programme that did not include performing protocol biopsies after the first confirmatory biopsy.
Patients and Methods
All patients diagnosed with Gleason 3 + 3 prostate cancer because of a raised PSA level who underwent mpMRI after diagnosis were included. Patients were recorded in a prospective clinical database and followed up with PSA monitoring and repeat MRI. In patients who remained on active surveillance after the first MRI (with or without confirmatory biopsy), we investigated PSA dynamics for association with subsequent progression. Comparison between first and second MRI scans was undertaken. Outcomes assessed were: progression to radical therapy at first MRI/confirmatory biopsy and progression to radical therapy in those who remained on active surveillance after first MRI.
Results
A total of 211 patients were included, with a median of 4.2 years of follow‐up. The rate of progression to radical therapy was significantly greater at all stages among patients with visible lesions than in those with initially negative MRI (47/125 (37.6%) vs 11/86 (12.8%); odds ratio 4.1 (95% CI 2.0–8.5), P < 0.001). Only 1/56 patients (1.8%) with negative initial MRI scans who underwent a confirmatory systematic biopsy had upgrading to Gleason 3 + 4 disease. PSA velocity was significantly associated with subsequent progression in patients with negative initial MRI (area under the curve 0.85 [95% CI 0.75–0.94]; P <0.001). Patients with high‐risk visible lesions on first MRI who remained on active surveillance had a high risk of subsequent progression 19/76 (25.0%) vs 9/84 (10.7%) for patients with no visible lesions, despite reassuring targeted and systematic confirmatory biopsies and regardless of PSA dynamics.
Conclusion
Men with low‐risk Gleason 3 + 3 prostate cancer on active surveillance can forgo protocol biopsies in favour of MRI and PSA monitoring with selective re‐biopsy.
Editorial: Re‐thinking active surveillance for the multiparametric magnetic resonance imaging era
The last decade has seen a dramatic change in the management of low‐risk prostate cancer. Active surveillance (AS) has moved from a controversial management strategy to the preferred option for men with low‐risk disease. Despite widespread acceptance, there remain aspects of the pathway that men find difficult to accept, including the need for numerous repeat surveillance biopsies. In this issue of the BJUI, Gallagher et al. [1] report the outcomes of an AS programme using selective repeat biopsy based on multiparametric MRI (mpMRI) and PSA dynamics. The authors address the important issue of whether mpMRI can be used to safely avoid repeat biopsies in AS protocols.
The evidence for repeat biopsies in AS is based on studies from the pre‐MRI era, where up to 30% of men were upgraded on repeat systematic TRUS biopsy [2]. It has been established that TRUS biopsy is a highly unreliable test and misses a substantial proportion of clinically significant disease. The current approach requiring the repeated application of an unreliable test will not improve the systematic error inherent to the test. It is clear that the pathway needs to be updated for the mpMRI era, and the cohort of men in Gallagher et al. [1] provides valuable real‐life clinical data of an mpMRI‐based AS programme with a unique 4‐year follow‐up period.
The results are encouraging, with upgrading occurring in only 1.8% of men with a prior negative MRI. With follow‐up, progression to radical treatment was 12.8%, which is consistent with the established diagnostic performance of mpMRI. The authors seek further improvements by investigating if PSA dynamics can identify men with a negative MRI at risk of progression. They find that PSA velocity is strongly associated with subsequent progression (AUC 0.95, P < 0.001) and conclude that men on AS with low‐risk disease can safely avoid biopsy in favour of MRI, PSA monitoring and selective re‐biopsy. This study [1] supports a growing body of evidence that mpMRI may be adopted as the primary surveillance tool for men on AS. The finding regarding PSA velocity should be interpreted carefully as it contrasts with previous studies, which found that PSA dynamics have a limited role as independent predictors of disease progressions in AS [3]. A non‐invasive alternative to biopsy would be a valuable addition to AS and improve its acceptability as a management option. The burden of repeat surveillance biopsies for men on AS should not be underestimated. Indeed, in the present study ~30% of men declined biopsy in favour of continued mpMRI surveillance. The question is can we adapt our current standard AS approach for the mpMRI era? There are still many challenges and many unanswered questions. The cost‐effectiveness of mpMRI surveillance programmes needs to be established and the lack of MRI capacity remains a significant obstacle in introducing mpMRI pathways. The optimal imaging interval and the natural history of mpMRI lesions are just a few of the questions that need further research. These are exciting times to be a researcher in this field and there is much work to do as we start to build the new evidence‐base covering all the questions required for the mpMRI era.
References
- Gallagher KM, Christopher E, Cameron AJ et al. Four‐year outcomes from a multiparametric magnetic resonance imaging (MRI)‐based active surveillance programme: PSA dynamics and serial MRI scans allow omission of protocol biopsies. BJU Int 2019; 123: 429–38.
- Dall’Era MA, Albertsen PC, Bangma C et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 2012; 62:976–83
- Loblaw A, Zhang L, Lam A et al. Comparing prostate specific antigen triggers for intervention in men with stable prostate cancer on active surveillance. J Urol 2010; 184: 1942–6
Article of the week: Does the introduction of prostate multi-parametric MRI into the AS protocol for localized PCa improve patient re-classification?
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
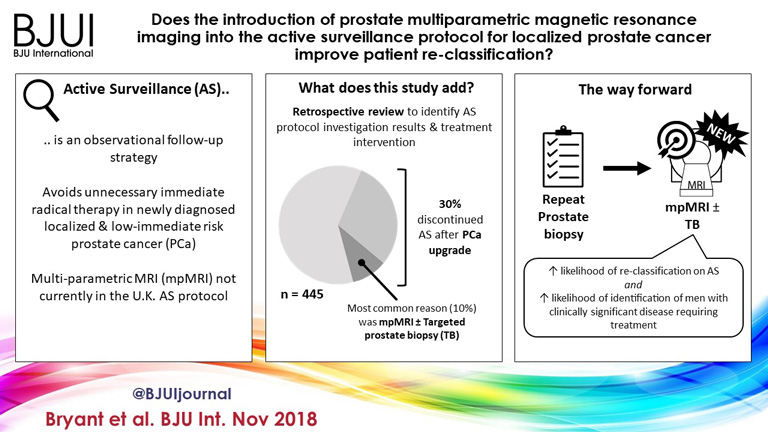
Does the introduction of prostate multiparametric magnetic resonance imaging into the active surveillance protocol for localized prostate cancer improve patient re-classification?
Richard J. Bryant*† , Bob Yang* , Yiannis Philippou*, Karla Lam*, Maureen Obiakor*, Jennifer Ayers*, Virginia Chiocchia†‡, Fergus Gleeson§, Ruth MacPherson§, Clare Verrill†¶, Prasanna Sooriakumaran†**, Freddie C. Hamdy*† and Simon F. Brewster*
*Department of Urology, Oxford University Hospitals NHS Foundation Trust, Oxford, UK, †Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK, ‡National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK, §Department of Radiology, Oxford University Hospitals NHS Foundation Trust, Oxford, UK, ¶Oxford NIHR Biomedical Research Centre, University of Oxford, Oxford, UK, and **Department of Uro-Oncology, University College London Hospital NHS Foundation Trust, London, UK
Abstract
Objectives
To determine whether replacement of protocol‐driven repeat prostate biopsy (PB) with multiparametric magnetic resonance imaging (mpMRI) ± repeat targeted prostate biopsy (TB) when evaluating men on active surveillance (AS) for low‐volume, low‐ to intermediate‐risk prostate cancer (PCa) altered the likelihood of or time to treatment, or reduced the number of repeat biopsies required to trigger treatment.
Patients and Methods
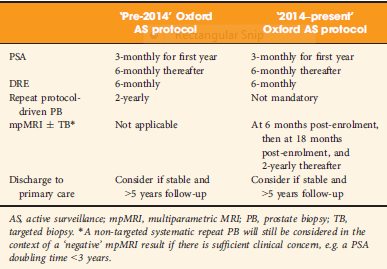
A total of 445 patients underwent AS in the period 2010–2016 at our institution, with a median (interquartile range [IQR]) follow‐up of 2.4 (1.2–3.7) years. Up to 2014, patients followed a ‘pre‐2014’ AS protocol, which incorporated PB, and subsequently, according to the 2014 National Institute for Health and Care Excellence (NICE) guidelines, patients followed a ‘2014–present’ AS protocol that included mpMRI. We identified four groups of patients within the cohort: ‘no mpMRI and no PB’; ‘PB alone’; ‘mpMRI ± TB’; and ‘PB and mpMRI ± TB’. Kaplan–Meier plots and log‐rank tests were used to compare groups.
Results
Of 445 patients, 132 (30%) discontinued AS and underwent treatment intervention, with a median (IQR) time to treatment of 1.55 (0.71–2.4) years. The commonest trigger for treatment was PCa upgrading after mpMRI and TB (43/132 patients, 29%). No significant difference was observed in the time at which patients receiving a PB alone or receiving mpMRI ± TB discontinued AS to undergo treatment (median 1.9 vs 1.33 years; P = 0.747). Considering only those patients who underwent repeat biopsy, a greater proportion of patients receiving TB after mpMRI discontinued AS compared with those receiving PB alone (29/66 [44%] vs 32/87 [37%]; P = 0.003). On average, a single set of repeat biopsies was needed to trigger treatment regardless of whether this was a PB or TB.
Conclusion
Replacing a systematic PB with mpMRI ±TB as part of an AS protocol increased the likelihood of re‐classifying patients on AS and identifying men with clinically significant disease requiring treatment. mpMRI ±TB as part of AS thereby represents a significant advance in the oncological safety of the AS protocol.
Article of the Month: MRI supported transperineal prostate biopsy
Every Month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy‐naïve men with suspicion of prostate cancer
Abstract
Objectives
To analyse the detection rates of primary magnetic resonance imaging (MRI)‐fusion transperineal prostate biopsy using combined targeted and systematic core distribution in three tertiary referral centres.
Patients and Methods
In this multicentre, prospective outcome study, 807 consecutive biopsy‐naïve patients underwent MRI‐guided transperineal prostate biopsy, as the first diagnostic intervention, between 10/2012 and 05/2016. MRI was reported following the Prostate Imaging‐Reporting and Data System (PI‐RADS) criteria. In all, 236 patients had 18–24 systematic transperineal biopsies only, and 571 patients underwent additional targeted biopsies either by MRI‐fusion or cognitive targeting if PI‐RADS ≥3 lesions were present. Detection rates for any and Gleason score 7–10 cancer in targeted and overall biopsy were calculated and predictive values were calculated for different PI‐RADS and PSA density (PSAD) groups.
Results
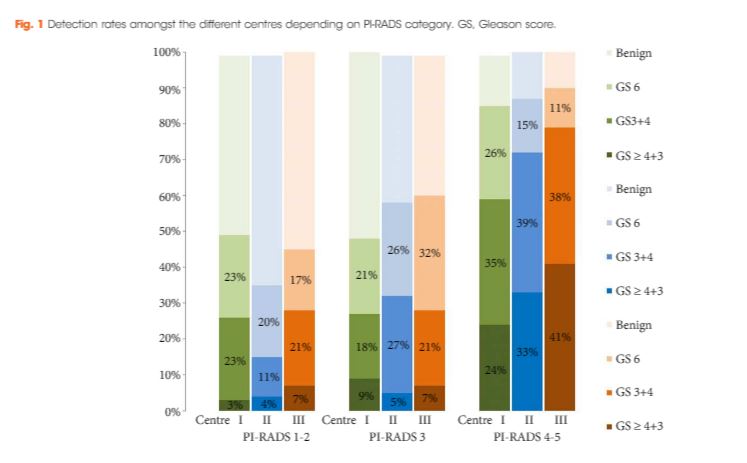
Cancer was detected in 68% of the patients (546/807) and Gleason score 7–10 cancer in 49% (392/807). The negative predictive value of 236 PI‐RADS 1–2 MRI in combination with PSAD of <0.1 ng/mL/mL for Gleason score 7–10 was 0.91 (95% confidence interval ± 0.07, 8% of study population). In 418 patients with PI‐RADS 4–5 lesions using targeted plus systematic biopsies, the cancer detection rate of Gleason score 7–10 was significantly higher at 71% vs 59% and 61% with either approach alone (P < 0.001). For 153 PI‐RADS 3 lesions, the detection rate was 31% with no significant difference to systematic biopsies with 27% (P > 0.05). Limitations include variability of multiparametric MRI (mpMRI) reading and Gleason grading.
Conclusion
MRI‐based transperineal biopsy performed at high‐volume tertiary care centres with a significant experience of prostate mpMRI and image‐guided targeted biopsies yielded high detection rates of Gleason score 7–10 cancer. Prostate biopsies may not be needed for men with low PSAD and an unsuspicious MRI. In patients with high probability lesions, combined targeted and systematic biopsies are recommended.
Editorial: Systematic transperineal and MRI‐targeted biopsies: the resolution of uncertainty
The paper published in this issue of the BJUI titled ‘Multicentre evaluation of magnetic resonance imaging supported transperineal biopsy in biopsy‐naïve men with suspicion of prostate cancer’ is timely and helps to resolve some of the uncertainty inherent within the diagnostic pathway 1.
The publication of the PROstate MRI Imaging Study (PROMIS) study, although demonstrating that 25% of patients might avoid prostate biopsy with a normal MRI (Prostate Imaging Reporting and Data System [PI‐RADS] 1–2) and that MRI could identify 90% of patients with high‐risk disease (PI‐RADS 5), did not resolve the issue of what to do with equivocal PI‐RADS 3 scans, uncertainty remained 2. The recent publication of the PRECISION trial (Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not?) has only contributed to the uncertainty of systematic TRUS biopsy and has shown that targeted biopsies resolve the issue for <50% of the patients overall and only 12% of those with PI‐RADS 3 lesions had a diagnosis of cancer on targeted biopsy only 3. The study has shown that in the face of an identifiable lesion a MRI‐targeted biopsy is non‐inferior to a blind systematic TRUS biopsy, which was positive in only 28% and implies that a systematic biopsy may be unnecessary, so where does that leave us? The uncertainty within MRI remains at the PI‐RADS 3 level, and particularly with a TRUS biopsy that is not a systematic biopsy of the peripheral zone. The authors of the paper highlighted in this issue of the BJUI 1 help to resolve the issue because they describe a more systematic biopsy.
The transperineal (TP) biopsy approach for systematic and targeted biopsy they use is that which was adopted by the Ginsburg Study Group on Enhanced Prostate Diagnostics 4. It is a systematic biopsy that preferentially targets the peripheral zone in a sectoral fashion. It avoids the oversampling inherent in template‐mapping biopsy and the under‐sampling of the non‐systematic transrectal biopsy. Their paper evaluates the combination of an MRI‐targeted biopsy with a systematic TP biopsy. It confirms, as suggested by the PROMIS study, that patients with PI‐RADS 1 or 2 prostates on MRI with a low PSA density <0.1 ng/mL/mL could safely avoid biopsy, based upon a negative predictive value of 0.91 on systematic biopsy. However, in 418 patients with PI‐RADS 4–5 lesions, it was the combination of a targeted and systematic TP biopsy that achieved an overall cancer detection rate of 71%, but that MRI‐targeted biopsies alone had a detection rate of 59% vs 61% for systematic TP biopsies. In the PI‐RAD 3 equivocal group the combined biopsy identified 30% with Gleason score 7–10, whereas targeted biopsy only was positive in 21% vs 27% with systematic biopsies.
The message is clear.
An appropriate systematic biopsy targeted to the peripheral zone remains an essential component of prostate diagnosis even in the MRI era, as indeed it did before MRI was available. In the pre‐MRI days, about one‐third of patients that had negative TRUS biopsies had cancer on TP biopsies and a third of those thought suitable for AS on TRUS biopsy had more significant disease. I suspect in the modern era that figure remains unchanged for those with PI‐RADS 1, 2 or 3, particularly with a PSA density >0.15 ng/mL/mL. As urologists we have always been criticised for over diagnosing and over treating prostate cancer but I suspect that the more heinous crime is that of under treatment of significant disease, it is the very reason why I started doing TP biopsies, to resolve uncertainty. I consider that MRI, for all its benefits in the diagnostic algorithm, cannot yet resolve that uncertainty.
Probably the only patients that merit a target‐only biopsy are those with the high‐PSA, large‐volume disease, easily visible on MRI and usually palpable. Prostate biopsy can be avoided or at least deferred in the PI‐RADS 1–2 group with low PSA density; the rest should be offered a systematic biopsy along with a targeted biopsy. This may be less important in those proceeding to whole gland treatment or surgical extirpation but remains essential in those considering active surveillance, brachytherapy, or any one of the myriad of unproven focal treatments becoming available. The authors should be congratulated for bringing some certainty to uncertainty.
- 1 Hansen NL, Barrett T, Kesch C et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy‐naïve men with suspicion of prostate cancer. BJU Int 2018; 122: 40–9
- 2 Ahmed HU, El‐Shater Bosaily A, Brown LC et al. Diagnostic accuracy of multi‐parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–22
- 3 Kasivisvanathan V, Ranniko A, Borghi M et al. MRI‐targeted or standard biopsy for prostate cancer diagnosis. N Engl J Med 2018. https://doi.org/10.1056/NEJMoa1801993 [Epub ahead of print]
- 4 Kuru TH, Wadhwa K, Chang RT et al. Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: a standardization approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU Int 2013; 112: 568–77
Article of the Week: Evaluation of targeted and systematic biopsies using MRI and US image-fusion guided transperineal prostate biopsy
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image-fusion guided transperineal prostate biopsy in patients with a previous negative biopsy
Abstract
Objectives
To evaluate the detection rates of targeted and systematic biopsies in magnetic resonance imaging (MRI) and ultrasound (US) image-fusion transperineal prostate biopsy for patients with previous benign transrectal biopsies in two high-volume centres.
Patients and Methods
A two centre prospective outcome study of 487 patients with previous benign biopsies that underwent transperineal MRI/US fusion-guided targeted and systematic saturation biopsy from 2012 to 2015. Multiparametric MRI (mpMRI) was reported according to Prostate Imaging Reporting and Data System (PI-RADS) Version 1. Detection of Gleason score 7–10 prostate cancer on biopsy was the primary outcome. Positive (PPV) and negative (NPV) predictive values including 95% confidence intervals (95% CIs) were calculated. Detection rates of targeted and systematic biopsies were compared using McNemar’s test.
Results
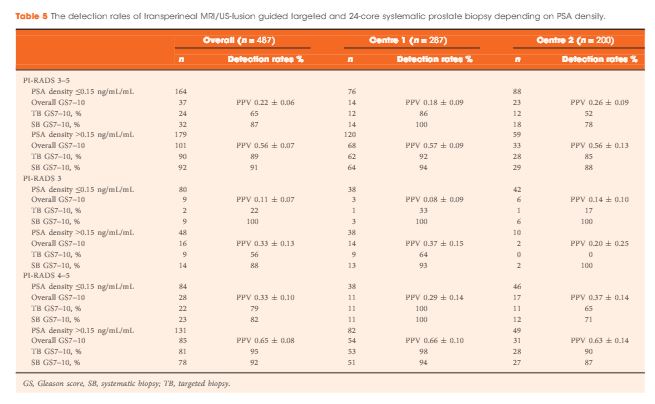
The median (interquartile range) PSA level was 9.0 (6.7–13.4) ng/mL. PI-RADS 3–5 mpMRI lesions were reported in 343 (70%) patients and Gleason score 7–10 prostate cancer was detected in 149 (31%). The PPV (95% CI) for detecting Gleason score 7–10 prostate cancer was 0.20 (±0.07) for PI-RADS 3, 0.32 (±0.09) for PI-RADS 4, and 0.70 (±0.08) for PI-RADS 5. The NPV (95% CI) of PI-RADS 1–2 was 0.92 (±0.04) for Gleason score 7–10 and 0.99 (±0.02) for Gleason score ≥4 + 3 cancer. Systematic biopsies alone found 125/138 (91%) Gleason score 7–10 cancers. In patients with suspicious lesions (PI-RADS 4–5) on mpMRI, systematic biopsies would not have detected 12/113 significant prostate cancers (11%), while targeted biopsies alone would have failed to diagnose 10/113 (9%). In equivocal lesions (PI-RADS 3), targeted biopsy alone would not have diagnosed 14/25 (56%) of Gleason score 7–10 cancers, whereas systematic biopsies alone would have missed 1/25 (4%). Combination with PSA density improved the area under the curve of PI-RADS from 0.822 to 0.846.
Conclusion
In patients with high probability mpMRI lesions, the highest detection rates of Gleason score 7–10 cancer still required combined targeted and systematic MRI/US image-fusion; however, systematic biopsy alone may be sufficient in patients with equivocal lesions. Repeated prostate biopsies may not be needed at all for patients with a low PSA density and a negative mpMRI read by experienced radiologists.
Editorial: Getting to the right biopsy in the right patient at the right time
Guidelines now recommend performing multiparametric MRI (mpMRI) and targeted prostate biopsies in men with a history of prior negative biopsy and continued concern for significant cancer. This new approach to prostate re-biopsy is aimed at improving prostate cancer detection. However, several important clinical factors may help clinicians’ fine-tune the process of repeated prostate biopsy. In this month’s issue of the BJUI, Hansen et al. [1] present a multicentre study of patients with prior negative TRUS biopsy undergoing MRI/TRUS-fusion transperineal biopsy.
In the study, 487 men undergo mpMRI and transperineal biopsy with detection of clinically significant (Gleason score 7–10) cancer as the primary outcome. Several factors are evaluated to compare cancer detection rates, including systematic biopsies, targeted biopsies, PSA density (PSAD), and Prostate Imaging Reporting and Data System (PI-RADS) version 1 score. From their cohort, a suspicious lesion (PIRADS 3–5) was identified in 343 (70%) patients. Prostate cancer was detected in 249 (51%), with 149 (31%) having Gleason score 7–10 cancer. Potentially missed significant cancers from the anterior prostate were found in 27% (40/149). Cancer was detected in 28% (40/144) of patients with PI-RADS 1–2 lesions, with 8% (11/144) being Gleason score 7–10. For patients with PI-RADS 3–5 lesions, cancer was identified in 61% (209/343) with 40% (138/343) being Gleason score 7–10. For patients with PI-RADS 3–5 lesions, systematic biopsies alone failed to detect 13/138 significant cancers, while targeted biopsies missed 24/138 cancers. The combination of systematic and targeted biopsies was significantly better for Gleason score 7–10 prostate cancer detection than either alone. The addition of a PSAD threshold of 0.15 ng/mL/mL for the detection of Gleason score 7–10 resulted in a significant improvement in the area under the curve (0.846) of the receiver operating characteristic curve for PSAD groups and PI-RADS score.
Getting the right biopsy: In this study [1], patients with a prior negative TRUS biopsy underwent TRUS-fusion transperineal biopsy. Having two approaches to prostate biopsy can be advantageous when evaluating men with prior negative biopsies. Historical studies have found comparable prostate cancer detection between transrectal and transperineal biopsies for men undergoing both initial biopsy [2] and saturation re-biopsy [3]. However, the detection of anterior lesions has remained a persistent challenge from the transrectal approach. As in the current study [1], use of transperineal biopsy can detect cancer in up to 30% of tumours that would otherwise be missed on extended template TRUS biopsy [4]. Although attempts to reach anterior lesions from the transrectal approach may be feasible [5], the transperineal approach is felt to provide better sampling in comparison [6].
Getting the right patient: Patient-specific factors such as PI-RADS lesions 3–5 and PSAD have become increasing utilised for stratifying patients who may benefit from additional biopsies using image guidance. As the authors suggest, patients with negative imaging may consider deferring repeat biopsy, particularly those with reassuring PSADs (<0.15 ng/mL/mL). In their study [1], only 4% (6/144) of men with negative mpMRI and a PSAD of <0.15 ng/mL/mL harboured clinically significant cancer (five Gleason score 3 + 4 and one Gleason score 8). Patients with concerning PSAD, but negative mpMRI and those with lesions identified in the peripheral zone could have the option to undergo repeated, fusion-directed TRUS or transperineal biopsy. For patients with lesions identified in the anterior prostate, a transperineal prostate biopsy may provide the highest detection rate.
At the right time: Now that high quality prostate MRI is becoming more widely available; men with a prior negative biopsy should strongly consider the benefit of repeated biopsy after prostate imaging. In addition to identifying suspicious lesions, calculating PSAD has been found to improve the likelihood of detecting clinically significant prostate cancer. Without additional testing, a personalised biopsy plan can be created.
A thorough discussion of the prescribed biopsy approach and the likelihood of detecting a significant cancer is the final step to the right biopsy in the right patient at the right time.