Posts
Article of the week: Likert vs PI‐RADS v2: a comparison of two radiological scoring systems for detection of clinically significant PCa
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community and a video prepared by the authors; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, we recommend this one.
Likert vs PI‐RADS v2: a comparison of two radiological scoring systems for detection of clinically significant prostate cancer
Christopher C. Khoo*†, David Eldred-Evans*†, Max Peters‡, Mariana Bertoncelli Tanaka*†, Mohamed Noureldin*†, Saiful Miah*†, Taimur Shah*†, Martin J. Connor*, Deepika Reddy*, Martin Clark§, Amish Lakhani§, Andrea Rockall§, Feargus Hosking-Jervis*, Emma Cullen*, Manit Arya*†, David Hrouda†, Hasan Qazi¶, Mathias Winkler*†, Henry Tam§ and Hashim U. Ahmed*†
*Imperial Prostate, Division of Surgery, Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, †Imperial Urology, Charing Cross Hospital, Imperial College Healthcare NHS Trust, London, UK, ‡Department of Radiotherapy, University Medical Centre, Utrecht, The Netherlands, §Department of Radiology, Charing Cross Hospital, Imperial College Healthcare NHS Trust and ¶Department of Urology, St. George’s Hospital, St. George’s Healthcare NHS Trust, London, UK
Abstract
Objective
To compare the clinical validity and utility of Likert assessment and the Prostate Imaging Reporting and Data System (PI‐RADS) v2 in the detection of clinically significant and insignificant prostate cancer.
Patients and Methods
A total of 489 pre‐biopsy multiparametric magnetic resonance imaging (mpMRI) scans in consecutive patients were subject to prospective paired reporting using both Likert and PI‐RADS v2 by expert uro‐radiologists. Patients were offered biopsy for any Likert or PI‐RADS score ≥4 or a score of 3 with PSA density ≥0.12 ng/mL/mL. Utility was evaluated in terms of proportion biopsied, and proportion of clinically significant and insignificant cancer detected (both overall and on a ‘per score’ basis). In those patients biopsied, the overall accuracy of each system was assessed by calculating total and partial area under the receiver‐operating characteristic (ROC) curves. The primary threshold of significance was Gleason ≥3 + 4. Secondary thresholds of Gleason ≥4 + 3, Ahmed/UCL1 (Gleason ≥4 + 3 or maximum cancer core length [CCL] ≥6 or total CCL≥6) and Ahmed/UCL2 (Gleason ≥3 + 4 or maximum CCL ≥4 or total CCL ≥6) were also used.
Table 1: Comparison of Likert and Prostate Imaging Reporting and Data System scoring.
Results
The median (interquartile range [IQR]) age was 66 (60–72) years and the median (IQR) prostate‐specific antigen level was 7 (5–10) ng/mL. A similar proportion of men met the biopsy threshold and underwent biopsy in both groups (83.8% [Likert] vs 84.8% [PI‐RADS v2]; P = 0.704). The Likert system predicted more clinically significant cancers than PI‐RADS across all disease thresholds. Rates of insignificant cancers were comparable in each group. ROC analysis of biopsied patients showed that, although both scoring systems performed well as predictors of significant cancer, Likert scoring was superior to PI‐RADS v2, exhibiting higher total and partial areas under the ROC curve.
Conclusions
Both scoring systems demonstrated good diagnostic performance, with similar rates of decision to biopsy. Overall, Likert was superior by all definitions of clinically significant prostate cancer. It has the advantages of being flexible, intuitive and allowing inclusion of clinical data. However, its use should only be considered once radiologists have developed sufficient experience in reporting prostate mpMRI.
Editorial: Does prostate MRI reporting system affect performance of MRI in men with a clinical suspicion of PCa?
Magnetic Resonance Imaging (MRI) of prostate continues to transform the way prostate cancer is being diagnosed and risk stratified. Multiple prospective single (e.g. the Biparametric MRI for Detection of Prostate Cancer [BIDOC] [1] and Improved Prostate Cancer Diagnosis ‐ Combination of Magnetic Resonance Imaging and Biomarkers [IMPROD] [2]) and multi‐institution trials (e.g. PROstate MRI Imaging Study [PROMIS] [3], PRostate Evaluation for Clinically Important Disease: Sampling Using Image‐guidance Or Not? [PRECISION] [4], multi‐institutional IMPROD (Multi‐IMPROD) [5], Assessment of Prostate MRI Before Prostate Biopsies [MRI‐FIRST] [6]) have demonstrated the potential of prostate MRI to limit the number of unnecessary biopsies in men with suspected prostate cancer.
In this issue of the BJUI, Khoo et al. [7] retrospectively analysed reports from a multicentre prostate cancer pathway registry, Rapid Assessment and Prostate Imaging for Diagnosis (RAPID). Men with a clinical suspicion of prostate cancer were enrolled based on various clinical criteria such as: age, performance status, and PSA level. All men had a pre‐biopsy MRI, including dynamic contrast‐enhanced MRI, reported using a 5‐point Likert scale and Prostate Imaging Reporting and Data System version 2.0 (PI‐RADSv2.0) systems by one of four uro‐radiologists (5–9 years of experience of prostate multi‐parametric MRI). Subsequently, all Likert and PI‐RADSv2.0 scores were reviewed by a dedicated reader in a multidisciplinary team setting. Likert scores were reported with knowledge of clinical variables such as: PSA, patient age, and past medical history. Men with Likert or PI‐RADSv2.0 score ≥4 or a score of 3 with a PSA density ≥0.12 ng/mL/mL underwent transperineal targeted prostate biopsies. Additionally, some men below these thresholds deemed to be at particularly high risk of prostate cancer (usually based on presence of other risk factors such as family history, high PSA kinetics or ethnic risk) were also offered biopsy on a case‐by‐case basis. At least three targeted cores were taken from each MRI‐suspicious lesion and no systematic biopsy cores were included in this analysis.
In total, 489 men were included in the analyses, with 377 and 408 men meeting the Likert and PI‐RADSv2.0 biopsy thresholds, respectively, of whom 316 (83.8%) and 346 (84.8%) proceeded to biopsy (P = 0.704), respectively. The Likert system predicted more clinically significant prostate cancer than PI‐RADSv2.0, e.g., 58.2% (184/316) vs 53.2% (184/346) of prostate cancer (P = 0.190) with Gleason score ≥3+4. Detection rates of clinically insignificant prostate cancer were comparable. The authors concluded that the Likert system was superior to PI‐RADSv2.0.
The authors should be congratulated on their effort to improve prostate MRI as a risk‐stratification and biopsy targeting tool. However, caution should be applied when translating these results to other centres. In order to access inter‐centre variability and to allow independent external validation, research groups should provide access to their imaging and patient level data. The authors do not provide such access and do not present inter‐reader variability of Likert vs PI‐RADv2.0 for all enrolled men. Similar to other trials evaluating prostate MRI in men with a clinical suspicion of prostate cancer, true prostate cancer and significant prostate cancer prevalence in this cohort is unknown, as men did not undergo saturation biopsy or prostatectomy with whole‐mount prostatectomy sections.
Overall, this retrospective analysis by Khoo et al. [7], comparing Likert scores reported using clinical variables vs PIRADSv2.0, provides further evidence that good quality prostate MRI can be used as a risk‐stratification and biopsy targeting tool in men with a clinical suspicion of prostate cancer. Each centre needs to develop its own quality control process and continually review its own performance measures of prostate MRI and MRI‐targeted biopsy. Furthermore, in order to access inter‐centre variability in performance of prostate MRI and MRI‐targeted biopsy, free public access to imaging and patient level data should be provided.
by Ivan Jambor and Ugo Falagorio
References
- , , et al. Assessment of the diagnostic accuracy of biparametric magnetic resonance imaging for prostate cancer in biopsy‐naive men: the Biparametric MRI for Detection of Prostate Cancer (BIDOC) study. JAMA Netw Open 2018; 1: 1– 28
- , , et al. Novel biparametric MRI and targeted biopsy improves risk stratification in men with a clinical suspicion of prostate cancer (IMPROD Trial). J Magn Reson Imaging 2017; 46: 1089– 95
- , , et al. Diagnostic accuracy of multi‐parametric MRI and TRUS Biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815– 22
- , , et al. MRI‐targeted or standard biopsy for prostate‐cancer diagnosis. N Engl J Med 2018; 378: 1767– 77
- , , et al. Validation of IMPROD biparametric MRI in men with clinically suspected prostate cancer: A prospective multi‐institutional trial. PLoS Med 2019; 16: e1002813.
- , , et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy‐naive patients (MRI‐FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 2019; 20: 100– 9
- , , et al. Likert vs PI‐RADS v2: a comparison of two radiological scoring systems for detection of clinically significant prostate cancer. BJU Int 2019; 125:49-55.
Video: Likert vs PI-RADS v2
Likert vs PI‐RADS v2: a comparison of two radiological scoring systems for detection of clinically significant prostate cancer
Abstract
Objective
To compare the clinical validity and utility of Likert assessment and the Prostate Imaging Reporting and Data System (PI‐RADS) v2 in the detection of clinically significant and insignificant prostate cancer.
Patients and Methods
A total of 489 pre‐biopsy multiparametric magnetic resonance imaging (mpMRI) scans in consecutive patients were subject to prospective paired reporting using both Likert and PI‐RADS v2 by expert uro‐radiologists. Patients were offered biopsy for any Likert or PI‐RADS score ≥4 or a score of 3 with PSA density ≥0.12 ng/mL/mL. Utility was evaluated in terms of proportion biopsied, and proportion of clinically significant and insignificant cancer detected (both overall and on a ‘per score’ basis). In those patients biopsied, the overall accuracy of each system was assessed by calculating total and partial area under the receiver‐operating characteristic (ROC) curves. The primary threshold of significance was Gleason ≥3 + 4. Secondary thresholds of Gleason ≥4 + 3, Ahmed/UCL1 (Gleason ≥4 + 3 or maximum cancer core length [CCL] ≥6 or total CCL≥6) and Ahmed/UCL2 (Gleason ≥3 + 4 or maximum CCL ≥4 or total CCL ≥6) were also used.
Results
The median (interquartile range [IQR]) age was 66 (60–72) years and the median (IQR) prostate‐specific antigen level was 7 (5–10) ng/mL. A similar proportion of men met the biopsy threshold and underwent biopsy in both groups (83.8% [Likert] vs 84.8% [PI‐RADS v2]; P = 0.704). The Likert system predicted more clinically significant cancers than PI‐RADS across all disease thresholds. Rates of insignificant cancers were comparable in each group. ROC analysis of biopsied patients showed that, although both scoring systems performed well as predictors of significant cancer, Likert scoring was superior to PI‐RADS v2, exhibiting higher total and partial areas under the ROC curve.
Conclusions
Both scoring systems demonstrated good diagnostic performance, with similar rates of decision to biopsy. Overall, Likert was superior by all definitions of clinically significant prostate cancer. It has the advantages of being flexible, intuitive and allowing inclusion of clinical data. However, its use should only be considered once radiologists have developed sufficient experience in reporting prostate mpMRI.
Article of the week: mpMRI and follow‐up to avoid prostate biopsy in 4259 men
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community and a video prepared by the authors. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Multiparametric magnetic resonance imaging and follow‐up to avoid prostate biopsy in 4259 men
Wulphert Venderink*, Annemarijke van Luijtelaar*, Marloes van der Leest*, Jelle O. Barentsz*, Sjoerd F.M. Jenniskens*, Michiel J.P. Sedelaar†,Christina Hulsbergen-van de Kaa‡, Christiaan G. Overduin* and Jurgen J. Fütterer*
*Department of Radiology and Nuclear Medicine, †Department of Urology, and ‡Department of Pathology, Radboud University Medical Center, Nijmegen, the Netherlands
Abstract
Objective
To determine the proportion of men avoiding biopsy because of negative multiparametric magnetic resonance imaging (mpMRI) findings in a prostate MRI expert centre, and to assess the number of clinically significant prostate cancers (csPCa) detected during follow‐up.
Patients and method
Retrospective study of 4259 consecutive men having mpMRI of the prostate between January 2012 and December 2017, with either a history of previous negative transrectal ultrasonography‐guided biopsy or biopsy naïve. Patients underwent mpMRI in a referral centre. Lesions were classified according to Prostate Imaging Reporting And Data System (PI‐RADS) versions 1 and 2. Negative mpMRI was defined as an index lesion PI‐RADS ≤2. Follow‐up until 13 October 2018 was collected by searching the Dutch Pathology Registry (PALGA). Gleason score ≥3 + 4 was considered csPCa. Kaplan–Meier analysis and univariable logistic regression models were used in the cohort of patients with negative mpMRI and follow‐up.
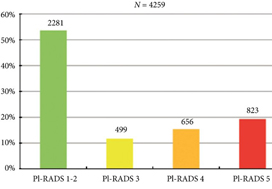
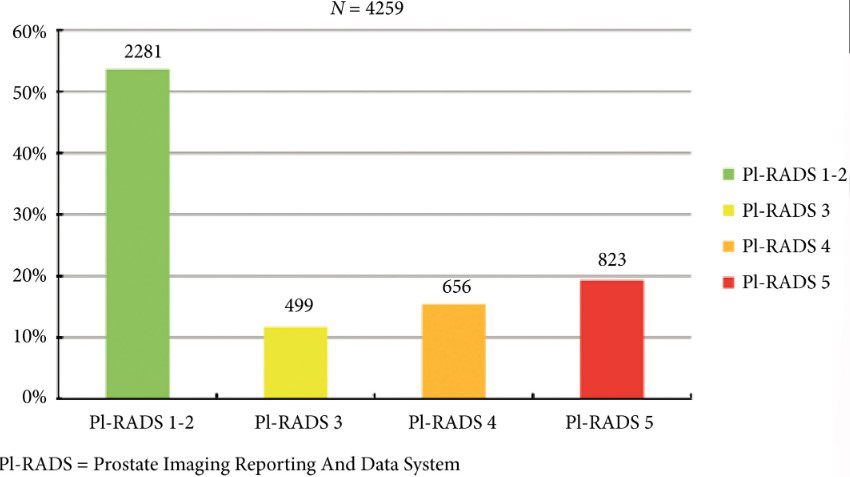
Fig. 2. Distribution of PI‐RADS scored in the entire cohort.
Results
Overall, in 53.6% (2281/4259) of patients had a lesion classified as PI‐RADS ≤2. In 320 patients with PI‐RADS 1 or 2, follow‐up mpMRI was obtained after a median (interquartile range) of 57 (41–63) months. In those patients, csPCa diagnosis‐free survival (DFS) was 99.6% after 3 years. Univariable logistic regression analysis revealed age as a predictor for csPCa during follow‐up (P < 0.05). In biopsied patients, csPCa was detected in 15.8% (19/120), 43.2% (228/528) and 74.5% (483/648) with PI‐RADS 3, 4 and 5, respectively.
Conclusion
More than half of patients having mpMRI of the prostate avoided biopsy. In those patients, csPCa DFS was 99.6% after 3 years.
Editorial: Avoiding biopsy in men with PI‐RADS scores 1 and 2 on mpMRI of the prostate, ready for prime time?
In 2019 is it safe to avoid prostate biopsy in men with Prostate Imaging Reporting and Data System (PI‐RADS) score 1 and 2 lesions reported on their multiparametric MRI (mpMRI)? In this journal, Venderink et al. [1] suggest that more than half the men being investigated for suspected prostate cancer could indeed safely avoid an initial biopsy. However, like other investigators in this field, the authors make an assumption in their study that there is such a paucity of clinically significant cancer in men with PI‐RADS 1 and 2 lesions, that biopsy is not deemed necessary, as in the PRECISION study [2]. In this study [1] from the Netherlands, of the 2281 men with an initial diagnosis of PI‐RADS 1 or 2 lesions, only 320 men had follow‐up mpMRI, and biopsies were only performed in a small number of men with PI‐RADS scores ≥ 3. Whilst one could conclude that 84% of men did not progress, based on serial imaging, one cannot prove what may have been missed.
Comparing mpMRI of the prostate to the reference standard of radical prostatectomy whole‐mount specimens, a study from the University of California, Los Angeles showed that mpMRI can potentially miss up to 35% of clinically significant cancers, and up to 20% of high grade cancers. It found that 74% of missed solitary tumours were clinically significant, including 23% with Gleason ≥4 + 3 = 7, and that 38.7% were >1 cm in diameter [3]. As such, these missed cancers were not all small, low grade and clinically insignificant. An Italian study confirmed these findings with a detection rate of clinically significant prostate cancer outside the index lesion seen on mpMRI in 30% of patients [4]. All urologists are aware that biopsy by any means can never detect all the cancers seen on formal whole‐mount histopathology, but we do have evidence using transperineal prostate mapping biopsies as the reference standard as to what may be missed. The PROMIS study [5] provides the best evidence using several definitions of clinically significant cancer. Using Gleason ≥4 + 3 or cancer core length >6 mm the negative predictive value (NPV) of a negative mpMRI was 89%. However, if the criteria were altered to any Gleason 7 cancer, the NPV falls to 76%. This is also supported by a multicentre study by Hansen et al. [6], which demonstrated that the NPV of a negative mpMRI for excluding Gleason 7–10 cancer was 80%, but improved to 91% with a PSA density of <0.1 ng/mL/mL, and to 89% with a PSA density of <0.15 ng/mL/mL. It is important to note that these studies used transperineal biopsies rather than 12‐core transrectal biopsies, suggesting the latter to be a more unreliable reference test with a greater probability of missing clinically significant cancer on systematic sampling.
Are all Gleason 3 + 4 = 7 cancers < 6 mm in core length, for example, 5 mm Gleason 3 + 4 (40%) = 7 cancer, truly clinically insignificant? If that were the case, favourable intermediate‐risk prostate cancer would have to be an accepted indication for active surveillance (AS) in men, and in most cases this is not the case. National Comprehensive Cancer Network guidelines recommend that men with favourable intermediate‐risk prostate cancer should only be offered AS if the PSA is <10 ng/mL, the lesion is cT1 and the percentage of positive cores is <50%. Prostate Cancer Research International Active Surveillance (PRIAS) criteria only accept men with favourable intermediate‐risk prostate cancer if there is a maximum of two cores involved, PSA density is <0.2 ng/mL/mL, and if it represents <10% of the core. Both European Association of Urology and AUA guidelines caution that if men are offered AS with favourable intermediate‐risk disease, they should be warned of the greater risk of developing metastatic spread. It is therefore clear that major international guidelines do not fully support AS for intermediate‐risk prostate cancers and therefore it may not be acceptable to be missing Gleason 3 + 4 cancers in up to 10–20% of men with normal prostate mpMRI results.
Multiparametric MRI of the prostate has been a huge advance in prostate cancer diagnostics and is now widely used internationally, but does have limitations. Based on the available data, men who choose not to be biopsied with a normal prostate mpMRI should be warned, as part of informed consent, that a clinically significant cancer could be missed in up to 10–20% of cases (depending on PSA density) and close follow‐up should be recommended. One could easily argue that men with normal prostate mpMRI but with PSA density >0.15 ng/mL/mL should still be offered a systematic biopsy. Perhaps the future lies in the genomics of mpMRI‐visible vs ‐invisible lesions, with a recent study showing that there is a confluence of aggressive molecular and pathological features in lesions visible on MRI. Future research may be able to determine if indeed it is safe to leave some Gleason 3 + 4 = 7 cancers undetected if invisible on mpMRI because of their lack of genomic and metabolic aggression rather than based on their Gleason pattern [7].
by Mark Frydenberg
References
- , , et al. Multiparametric MRI and follow up to avoid prostate biopsy in 4259 men. BJU Int 2019; 124: 775– 84
- , , et al. MRI targeted or standard biopsy for prostate cancer diagnosis. N Engl J Med 2018; 378: 1767– 77
- , , et al. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol 2019; 75: 712– 20
- , , et al. Association between prostate Imaging Reporting and data system (PIRADS) score for the index lesion and multifocal clinically significant prostate cancer. Eur Urol Oncol 2018; 1: 29– 3336
- , , et al. Diagnostic accuracy of multiparametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815– 22
- , , et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy naïve men with suspicion of prostate cancer. BJU Int 2018; 122: 40– 9
- , , et al. Molecular hallmarks of multiparametric magnetic resonance imaging visibility in prostate cancer. Eur Urol 2019; 76: 18– 23
Video: mpMRI and follow-up to avoid prostate biopsy in 4259 men
Multiparametric magnetic resonance imaging and follow-up to avoid prostate biopsy in 4259 men
Abstract
Objective
To determine the proportion of men avoiding biopsy because of negative multiparametric magnetic resonance imaging (mpMRI) findings in a prostate MRI expert centre, and to assess the number of clinically significant prostate cancers (csPCa) detected during follow‐up.
Patients and methods
Retrospective study of 4259 consecutive men having mpMRI of the prostate between January 2012 and December 2017, with either a history of previous negative transrectal ultrasonography‐guided biopsy or biopsy naïve. Patients underwent mpMRI in a referral centre. Lesions were classified according to Prostate Imaging Reporting And Data System (PI‐RADS) versions 1 and 2. Negative mpMRI was defined as an index lesion PI‐RADS ≤2. Follow‐up until 13 October 2018 was collected by searching the Dutch Pathology Registry (PALGA). Gleason score ≥3 + 4 was considered csPCa. Kaplan–Meier analysis and univariable logistic regression models were used in the cohort of patients with negative mpMRI and follow‐up.
Results
Overall, in 53.6% (2281/4259) of patients had a lesion classified as PI‐RADS ≤2. In 320 patients with PI‐RADS 1 or 2, follow‐up mpMRI was obtained after a median (interquartile range) of 57 (41–63) months. In those patients, csPCa diagnosis‐free survival (DFS) was 99.6% after 3 years. Univariable logistic regression analysis revealed age as a predictor for csPCa during follow‐up (P < 0.05). In biopsied patients, csPCa was detected in 15.8% (19/120), 43.2% (228/528) and 74.5% (483/648) with PI‐RADS 3, 4 and 5, respectively.
Conclusion
More than half of patients having mpMRI of the prostate avoided biopsy. In those patients, csPCa DFS was 99.6% after 3 years.
Article of the week: Ultrasound characteristics of regions identified as suspicious by MRI predict the likelihood of clinically significant cancer on MRI–ultrasound fusion‐targeted biopsy
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community, and a video made by the authors. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
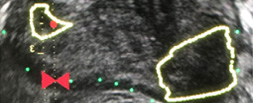
The ultrasound characteristics of regions identified as suspicious by magnetic resonance imaging (MRI) predict the likelihood of clinically significant cancer on MRI–ultrasound fusion‐targeted biopsy
Benjamin Press*, Andrew B. Rosenkrantz†, Richard Huang‡ and Samir S. Taneja§
*Rutgers New Jersey Medical School, Newark, NJ, †Department of Radiology, ‡Department of Urology, and §Departments of Urology and Radiology, NYU Langone Health, New York, NY, USA
Abstract
Objective
To determine whether the presence of an ultrasound hypoechoic region at the site of a region of interest (ROI) on magnetic resonance imaging (MRI) results in improved prostate cancer (PCa) detection and predicts clinically significant PCa on MRI–ultrasonography fusion‐targeted prostate biopsy (MRF‐TB).Materials and Methods
Between July 2011 and June 2017, 1058 men who underwent MRF‐TB, with or without systematic biopsy, by a single surgeon were prospectively entered into an institutional review board‐approved database. Each MRI ROI was identified and scored for suspicion by a single radiologist, and was prospectively evaluated for presence of a hypoechoic region at the site by the surgeon and graded as 0, 1 or 2, representing none, a poorly demarcated ROI‐HyR, or a well demarcated ROI‐HyR, respectively. The interaction of MRI suspicion score (mSS) and ultrasonography grade (USG), and the prediction of cancer detection rate by USG, were evaluated through univariate and multivariate analysis.Results
For 672 men, the overall and Gleason score (GS) ≥7 cancer detection rates were 61.2% and 39.6%, respectively. The cancer detection rates for USGs 0, 1 and 2 were 46.2%, 58.6% and 76.0% (P < 0.001) for any cancer, and 18.7%, 35.2% and 61.1% (P < 0.001) for GS ≥7 cancer, respectively. For MRF‐TB only, the GS ≥7 cancer detection rates for USG 0, 1 and 2 were 12.8%, 25.7% and 52.0%, respectively (P < 0.001). On univariate analysis, in men with mSS 2–4, USG was predictive of GS ≥7 cancer detection rate. Multivariable regression analysis showed that USG, prostate‐specific antigen density and mSS were predictive of GS ≥7 PCa on MRF‐TB.Conclusions
Ultrasonography findings at the site of an MRI ROI independently predict the likelihood of GS ≥7 PCa, as men with a well‐demarcated ROI‐HyR at the time of MRF‐TB have a higher risk than men without.Editorial: Is transrectal ultrasonography of the prostate obsolete in the MRI era?
Sampling of prostate tissue to confirm pathologically a clinical suspicion of cancer has undergone an exponential change. The random systematic prostate biopsy technique was the only method used for many decades, initially guided by the finger but, since 1989, performed with TRUS guidance. Now, within the space of only a few years, we have entered the era of performing prostate biopsies on the basis of high‐tech three‐dimensional multiparametric MRI images, including software that can track the exact course of the biopsy needle [1]. While new technical developments in general lead to better, more individually directed healthcare, there is always the risk of abandoning ‘old’ but well developed and extensively tested techniques too soon. In this issue of the BJUI, Press et al. [2] looked at the added value of the presence of an ‘old‐fashioned’ TRUS‐detected lesion in cancer‐suspicious regions on MRI to better predict the presence of clinically significant prostate cancer (csPCa) defined as Gleason score ≥7. In their study comprising 1058 men, it was shown that a well‐demarcated abnormal TRUS finding noted at the time of MRI‐TRUS fusion‐guided prostate biopsy coincides with an increased risk of csPCa detection, independent of MRI suspicion (Prostate Imaging Reporting and Data System [PI‐RADS] score).
Increasing PI‐RADS score is correlated with an increased percentage of csPCa after targeted biopsy, both at initial and repeat biopsy. In a review based on data from 8252 men, it was shown that there is a gradual increase in the detection of csPCa from PI‐RADS 3 to PI‐RADS 4 to PI‐RADS 5 index lesions. For example, at first biopsy, the overall rate of PCa detection and the percentage of csPCa were 39%, 62% and 92% and 54%, 63% and 76% for PI‐RADS 3, 4 and 5 lesions, respectively. This means that in men with PI‐RADS 3 lesions, representing approximately one‐third of men deemed eligible for further assessment, only 39% will be diagnosed with PCa and half of the PCa detected will be potentially indolent Gleason 6 PCa [3]. This makes this group of men extremely interesting for further risk stratification before biopsy. Multivariable risk stratification in which PSA density plays an important role has been shown to be of value in these men [4] but further refinement could potentially be made by including suspicious lesions identified at TRUS.
Apart from the added value of TRUS findings in terms of risk stratification, the performance of the MRI‐targeted biopsy itself could be improved by visual guidance of hypoechoic lesions. In the present study by Press et al [2], a hypoechoic TRUS lesion was present at or near the location of two‐thirds of cancer‐suspicious lesions on MRI. The authors more or less advise to direct the targeted biopsy cores not only to the MRI suspicious lesion, but also the TRUS suspicious lesion, both of which often do not fully overlay in a software‐assisted MRI‐TRUS fusion model. The extent to which this ‘correction for misregistration’ is already included during targeted biopsy in current clinical practice is unknown. Although feasible and seemingly important during software‐assisted fusion targeted biopsy, TRUS lesions in cancer‐suspicious MRI regions might be more frequently targeted during cognitive fusion‐targeted biopsy. Two recent studies underline the important message of the present study, and show that a considerable proportion of csPCa is missed in and around MRI‐suspicious lesions by targeted biopsies, as a result of sampling errors related to both misregistration and intra‐tumour heterogeneity [5, 6]. As suggested by these studies, visual guidance by hypoechoic lesions and ‘focal saturation’ biopsy by additional (peri‐)lesional cores might improve the detection of csPCa.
In summary, ‘good old’ TRUS could be of value in those patients who are virtually always present in scenarios in which a grading system is being used, i.e. patients belonging to the so‐called grey zone. The challenge of risk stratification (i.e. personalized medicine) is to nibble at both sides of the grey zone by implementing new techniques or, more likely by implementing a combination of all available and relevant knowledge.
by Monique J. Roobol, Frank-Jan H. Drost and Arnout R. Alberts
References
- , , et al. The current state of MR imaging‐targeted biopsy techniques for detection of prostate cancer. Radiology 2017; 285: 343–56
- , , , . The ultrasound characteristics of MRI suspicious regions predict the likelihood of clinically significant cancer on MRI‐ultrasound fusion targeted biopsy. BJUI 2019; 123: 439–46.
- . MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI‐RADS 3 lesions? Transl Androl Urol 2018; 7: 70–82
- , , , , , . Risk‐based patient selection for magnetic resonance imaging‐targeted prostate biopsy after negative transrectal ultrasound‐guided random biopsy avoids unnecessary magnetic resonance imaging scans. Eur Urol 2016; 69: 1129–34
- , , et al. Accuracy of transperineal targeted prostate biopsies, visual estimation and image fusion in men needing repeat biopsy in the PICTURE trial. J Urol 2018; 200: 1227–34
- Leest, M, , et al. Head‐to‐head comparison of transrectal ultrasound‐guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance‐guided biopsy in biopsy‐naive men with elevated prostate‐specific antigen: a large prospective multicenter clinical study. Eur Urol 2018; [Epub ahead of print]. https://doi.org/10.1016/j.eururo.2018.11.023.
Article of the Week: 3-Tesla mpMRI and TRUS-Bx in PCa patients on AS
Every week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Value of 3-Tesla multiparametric magnetic resonance imaging and targeted biopsy for improved risk stratification in patients considered for active surveillance
*, †, ‡, *, *, *, * and *
Departments of *Urology, †Diagnostic and Interventional Radiology, and ‡Department of Pathology, Instituto do Cancer, Universidade de Sao Paulo Faculdade de Medicina Hospital das Clinicas, Sao Paulo, SP, Brazil
Abstract
Objective
To evaluate the role of multiparametric magnetic resonance imaging (mpMRI) of the prostate and transrectal ultrasonography guided biopsy (TRUS-Bx) with visual estimation in early risk stratification of patients with prostate cancer on active surveillance (AS).
Patients and Methods
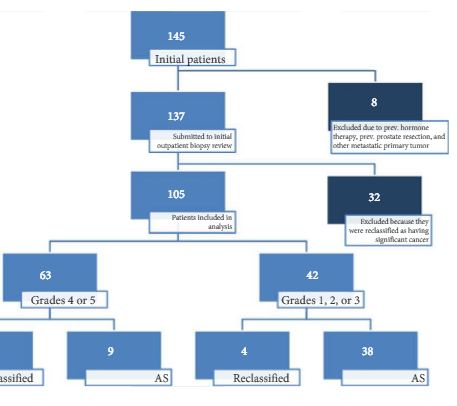
Patients with low-risk, low-grade, localised prostate cancer were prospectively enrolled and submitted to a 3-T 16-channel cardiac surface coil mpMRI of the prostate and confirmatory biopsy (CBx), which included a standard biopsy (SBx) and visual estimation-guided TRUS-Bx. Cancer-suspicious regions were defined using Prostate Imaging Reporting and Data System (PI-RADS) scores. Reclassification occurred if CBx confirmed the presence of a Gleason score ≥7, greater than three positive fragments, or ≥50% involvement of any core. The performance of mpMRI for the prediction of CBx results was assessed. Univariate and multivariate logistic regressions were performed to study relationships between age, prostate-specific antigen (PSA) level, PSA density (PSAD), number of positive cores in the initial biopsy, and mpMRI grade on CBx reclassification. Our report is consistent with the Standards of Reporting for MRI-targeted Biopsy Studies (START) guidelines.
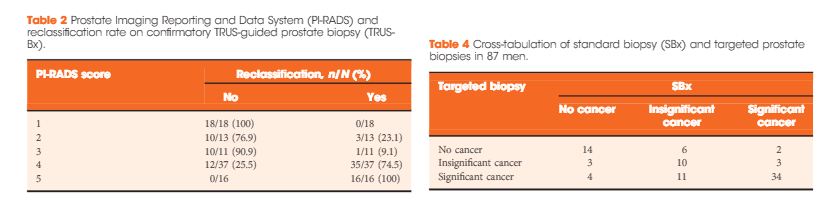
Results
In all, 105 patients were available for analysis in the study. From this cohort, 42 (40%) had PI-RADS 1, 2, or 3 lesions and 63 (60%) had only grade 4 or 5 lesions. Overall, 87 patients underwent visual estimation TRUS-Bx. Reclassification among patients with PI-RADS 1, 2, 3, 4, and 5 was 0%, 23.1%, 9.1%, 74.5%, and 100%, respectively. Overall, mpMRI sensitivity, specificity, positive predictive value, and negative predictive value for disease reclassification were 92.5%, 76%, 81%, and 90.5%, respectively. In the multivariate analysis, only PSAD and mpMRI remained significant for reclassification (P < 0.05). In the cross-tabulation, SBx would have missed 15 significant cases detected by targeted biopsy, but SBx did detect five cases of significant cancer not detected by targeted biopsy alone.
Conclusion
Multiparametric magnetic resonance imaging is a significant tool for predicting cancer severity reclassification on CBx among AS candidates. The reclassification rate on CBx is particularly high in the group of patients who have PI-RADS grades 4 or 5 lesions. Despite the usefulness of visual-guided biopsy, it still remains highly recommended to retrieve standard fragments during CBx in order to avoid missing significant tumours.