Posts
Podcast: NICE guideline on bedwetting in under 19s
Part of the BURST/BJUI podcast series
Tara Burnhope is a Specialty Registrar in Urology in the West Midlands Deanery and an active member of BURST.
Editorial: How long is long enough for pharmacological thromboprophylaxis in urology?
Each year, millions of patients who undergo urological surgery incur the risk of deep vein thrombosis and pulmonary embolism, together referred to as venous thromboembolism (VTE), and major bleeding. Because pharmacological prophylaxis decreases the risk of VTE, but increases the risk of bleeding, and because knowledge of the magnitude of these risks remains uncertain, both clinical practice and guideline recommendations vary widely [1]. One of the uncertainties is the recommended duration of pharmacological thromboprophylaxis.
In this issue of the BJUI, Naik et al. [2] provide an up‐to‐date review that summarises the articles that examined extended thromboprophylaxis in patients with cancer who underwent radical prostatectomy (RP), radical cystectomy (RC) or nephrectomy. The outcomes on which they focussed include risks of VTE, bleeding, renal failure and mortality – all potentially influenced by whether or not patients receive extended prophylaxis.
After screening >3500 articles, the authors included 18 studies, none of them randomised controlled trials (RCTs) [2]. They found that VTE risk is highest in open and robot‐assisted RC, and that, based on observational studies, extended thromboprophylaxis significantly reduces the risk of VTE relative to shorter duration prophylaxis. Evidence suggested that robot‐assisted RP, as well as both open and robot‐assisted partial and radical nephrectomies, incur lower VTE risk than RCs or open RP. They did not find studies comparing extended prophylaxis to standard prophylaxis for RPs or nephrectomies [2].
Overall, these findings are consistent with systematic reviews that estimated the procedure‐ and patient risk factor‐specific risks for 20 urological cancer procedures [3]. As these reviews suggested substantial procedure‐specific differences in the VTE risk estimates, the European Association of Urology (EAU) Guidelines provided separate recommendations for each procedure [4]. For urological (as well as gastrointestinal and gynaecological) patients, the National Institute for Health and Care Excellence (NICE) Guidelines suggest to ‘consider extending pharmacological VTE prophylaxis to 28 days postoperatively for people who have had major cancer surgery in the abdomen’ [5]. Because of variation in both bleeding and thrombosis risks across procedures, this advice is appropriate for some procedures and misguided for others. For instance, the procedure‐specific EAU Guidelines recommend extended VTE prophylaxis for open RC but not for robot‐assisted RP without lymphadenectomy [4].
The review by Naik et al. [2] identified the lack of urology‐specific studies comparing the in‐hospital‐only prophylaxis to extended prophylaxis. The few included studies were observational with considerable limitations (e.g. limited adjustment for possible confounders).
A recent update of a Cochrane review compared the impact of extended thromboprophylaxis with low‐molecular‐weight heparin (LMWH) for at least 14 days to in‐hospital‐only prophylaxis in abdominal or pelvic surgery procedures [6]. The authors identified seven RCTs (1728 participants) evaluating extended thromboprophylaxis with LMWH and generated pooled estimates for the incidence of any VTE (symptomatic or asymptomatic) after major abdominal or pelvic surgery of 13.2% in the control group compared with 5.3% in the patients receiving extended out‐of‐hospital LMWH (odds ratio [OR] 0.38, 95% CI 0.26–0.54).
Most events were asymptomatic, although the incidence of symptomatic VTE was also reduced from 1.0% in the in‐hospital‐only group to 0.1% in patients receiving extended thromboprophylaxis (OR 0.30, 95% CI 0.08–1.11). The authors reported no persuasive difference in the incidence of bleeding complications within 3 months of surgery (defined as major or minor bleeding according to the definition provided in the individual studies) between the in‐hospital‐only group (2.8%) and extended LMWH (3.4%) group (OR 1.10, 95% CI 0.67–1.81).
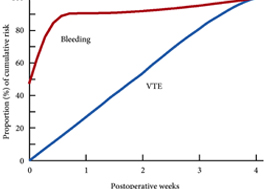
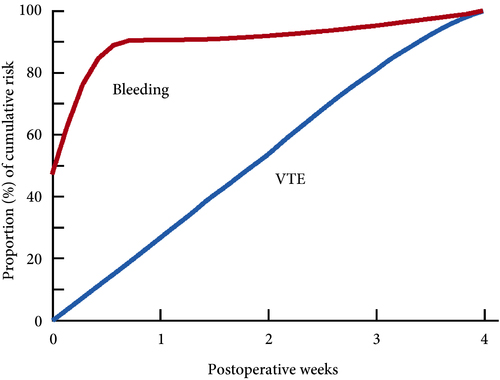
These findings are consistent with our own modelling study that demonstrated an approximately constant hazard of VTE up to 4 weeks after surgery [7]. That study also found that bleeding risk, by contrast, is concentrated in the first 4 days after surgery [7] (Fig.1). Using these findings, the EAU Guidelines suggest for patients in whom pharmacological prophylaxis is appropriate, extended pharmacological prophylaxis for 4 weeks [4]. Consistent with these recommendations, Naik et al. [2] found that 15 studies of 18 included in their review recommended extended prophylaxis.
Fig.1 Proportion of cumulative risk (%) of venous thromboembolism (VTE) and major bleeding by week since surgery during the first 4 postoperative weeks. Reproduced from: Tikkinen et al. [7].
Overall, as shown also by this review [2], the evidence base for urological thromboprophylaxis is limited. Although current evidence supports extended prophylaxis, definitively establishing the optimal duration of thromboprophylaxis will require large‐scale RCTs. Other unanswered key questions include: baseline risks of various procedures, timing of prophylaxis, patient risk stratification, as well as effectiveness of direct oral anticoagulants. In the meanwhile, suggesting extended duration to patients whose risk of VTE is sufficiently high constitutes a reasonable evidence‐based approach to VTE prophylaxis.
by Kari A.O. Tikkinen and Gordon H. Guyatt
References
- , , , , Guidelines of guidelines: thromboprophylaxis for urological surgery. BJU Int 2016; 118: 351– 8
- , , The role of extended venous thromboembolism prophylaxis for major urological cancer operations. BJU Int 2019; 124: 935-44
- , , et al. Procedure‐specific risks of thrombosis and bleeding in urological cancer surgery: systematic reviews and meta‐analyses. Eur Urol 2018; 73: 242– 51
- , , et al. EAU Guidelines on Thromboprophylaxis in Urological Surgery, 2017. European Association of Urology, 2018. Accessed November 2019
- National Institute for Health and Care Excellence (NICE). Venous Thromboembolism in over 16s: reducing the risk of hospital‐acquired deep vein thrombosis or pulmonary embolism. NICE guideline [NG89]. London: NICE, 2018. Accessed November 2019
- , , et al. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev 2019; 3: CD004318
- , , et al. Systematic reviews of observational studies of risk of thrombosis and bleeding in urological surgery (ROTBUS): introduction and methodology. Syst Rev 2014; 23: 150. DOI: 10.1186/2046‐4053‐3‐150.
Residents’ podcast: NICE guidelines: Urinary tract infection
Nikita Bhatt is a Specialist Trainee in Urology in the East of England Deanery and a BURST Committee member @BURSTUrology
NICE guideline: Urinary tract infection (lower): antimicrobial prescribing
This guideline sets out an antimicrobial prescribing strategy for lower urinary tract infection (also called cystitis) in children, young people and adults who do not have a catheter. It aims to optimise antibiotic use and reduce antibiotic resistance.
See also the following related NICE guidelines: Complicated UTIS; and Sepsis
BJUI Podcasts now available on iTunes, subscribe here https://itunes.apple.com/gb/podcast/bju-international/id1309570262
Article of the month: NICE Guidance – Prostate cancer: diagnosis and management
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, it should be this one.
NICE Guidance – Prostate cancer: diagnosis and management
Overview
This guideline covers the diagnosis and management of prostate cancer in secondary care, including information on the best way to diagnose and identify different stages of the disease, and how to manage adverse effects of treatment. It also includes recommendations on follow‐up in primary care for people diagnosed with prostate cancer.
Who is it for?
- Healthcare professionals
- Commissioners and providers of prostate cancer services
- People with prostate cancer, their families and carers
Context
Prostate cancer is the most common cancer in men, and the second most common cancer in the UK. In 2014, there were over 46,000 new diagnoses of prostate cancer, which accounts for 13% of all new cancers diagnosed. About 1 in 8 men will get prostate cancer at some point in their life. Prostate cancer can also affect transgender women, as the prostate is usually conserved after gender-confirming surgery, but it is not clear how common it is in this population.
More than 50% of prostate cancer diagnoses in the UK each year are in men aged 70 years and over (2012), and the incidence rate is highest in men aged 90 years and over (2012 to 2014). Out of every 10 prostate cancer cases, 4 are only diagnosed at a late stage in England (2014) and Northern Ireland (2010 to 2014). Incidence rates are projected to rise by 12% between 2014 and 2035 in the UK to 233 cases per 100,000 in 2035.
A total of 84% of men aged 60 to 69 years at diagnosis in 2010/2011 are predicted to survive for 10 or more years after diagnosis. When diagnosed at the earliest stage, virtually all people with prostate cancer survive 5 years or more: this is compared with less than a third of people surviving 5 years or more when diagnosed at the latest stage.
There were approximately 11,000 deaths from prostate cancer in 2014. Mortality rates from prostate cancer are highest in men aged 90 years and over (2012 to 2014). Over the past decade, mortality rates have decreased by more than 13% in the UK. Mortality rates are projected to fall by 16% between 2014 and 2035 to 48 deaths per 100,000 men in 2035.
People of African family origin are at higher risk of prostate cancer (lifetime risk of approximately 1 in 4). Prostate cancer is inversely associated with deprivation, with a higher incidence of cases found in more affluent areas of the UK.
Costs for the inpatient treatment of prostate cancer are predicted to rise to £320.6 million per year in 2020 (from
£276.9 million per year in 2010).
This guidance was updated in 2014 to include several treatments that have been licensed for the management of
hormone-relapsed metastatic prostate cancer since the publication of the original NICE guideline in 2008.
Since the last update in 2014, there have been changes in the way that prostate cancer is diagnosed and treated. Advances in imaging technology, especially multiparametric MRI, have led to changes in practice, and new evidence about some prostate cancer treatments means that some recommendations needed to be updated.
Editorial: NICE guidelines on prostate cancer 2019
The much‐anticipated National Institute for Health and Care Excellence (NICE) Guidelines are finally published [1] after a period of consultation when they were in the draft phase. These are updated from the previous 2008 and 2014 versions and reflect the changes in our knowledge and practice over the last 10 years. While there are many similarities, the astute reader will find distinct differences from the AUA Guidelines, which feature in a summary booklet released at the #AUA19 meeting in Chicago this spring.
NICE does not comment on screening for prostate cancer so many of us continue to rely on our Guideline of Guidelines [2], which make pragmatic recommendations such as smart screening in well‐informed men who are at higher risk because of their family history. For staging, bone scan has not been replaced by prostate‐specific membrane antigen (PSMA)‐positron‐emission tomography/CT, and Lu‐PSMA theranostics is yet to become an option in castrate‐resistant disease as the international trials are not mature.
Multiparametric MRI before prostate biopsy in men suitable for radical treatment is a new addition, based on the PROMIS [3] and PRECISION trials [1]. This approach is thought to be cost‐effective through reducing the number of biopsies and side effects despite the initial added cost of MRI scanning. In Grade Group 1 and some low‐volume Grade Group 2 cancers, protocol‐based active surveillance is recommended provided the patients are well counselled and it has been discussed by a multidisciplinary team.
To reduce variations in active surveillance, Prostate Cancer UK has carefully examined eight different guidelines and published a consensus statement for the benefit of our patients [4]. We have already promoted this widely on social media and hope that our readers will use this practical tool in their clinics. We often find that some patients just cannot live with a cancer inside their body and seek surgery as a result, however small their tumour. Careful discussion about management options and their risks vs benefits [1] can help patients arrive at a pragmatic decision. The effect of a cancer diagnosis on patients’ minds should therefore not be underestimated and a trained psychologist should be available for appropriate counselling.
NICE also recommends hypofractionated intensity‐modulated radiotherapy, if appropriate, in combination with androgen deprivation therapy (ADT) for localized disease, and methods of decreasing the side effects while increasing accuracy of radiation. As in 2014, robot‐assisted radical prostatectomy remains a surgical option in centres performing at least 150 of these procedures per year [1]. These numbers are similar to those published from other health services such as Canada. One such very high‐volume centre is the Martini Clinic which has reported its comparison of open and robot‐assisted radical prostatectomy in >10 000 patients. The oncological and functional outcomes are no different, open surgery is quicker and there is less blood loss and shorter time to catheter removal after robotic surgery. Just like the randomized trial of the two techniques, this large series highlights that surgeon experience rather than the technique is more important for clinical outcomes [5]. Finally, based on the STAMPEDE results, docetaxel is recommended for metastasis in addition to ADT and can be considered for high‐risk patients receiving ADT and radiotherapy [6]. NICE has also identified a number of important research questions which we hope will be answered by ongoing studies in coming years.
by Prokar Dasgpta, John Davis & Simon Hughes
References
- NICE Guidance. NICE guidelines prostate cancer. BJU Int 2019; 124: 9– 26.
- . Review of prostate cancer screening guidelines. BJU Int 2014; 114: 323– 5
- . The PROMIS of MRI. BJU Int 2016; 118: 7
- , , et al. PCUK consensus statement. BJUI 2019; 124: 47– 54
- , , et al. A comparative study of robot‐assisted and open radical prostatectomy in 10 790 men treated by highly trained surgeons for both procedures. BJU Int 2019; 123: 1031– 40
- , , et al. Taxane‐based chemohormonal therapy for metastatic hormone‐sensitive prostate cancer: a Cochrane Review. BJU Int 2019; [Epub ahead of print]. https://doi.org/10.1111/bju.14711
Residents’ podcast: NICE guidelines bladder cancer
Part of the BURST (@BURSTurology) series of Residents’ podcasts.
Mr Daniel Beder is a BURST Core Surgical Trainee.
Read the article here: NICE guidelines bladder cancer
NICE Stone Guidelines 2019
The NICE (National Institute For Health And Care Excellence) “Renal and ureteric stones: assessment and management” guideline NG118 was published on-line on Tuesday 8th January 2019 and appeared on the BJUI website on Friday 18th January.
NICE guidelines are based on the best available evidence for the treatment of the specific clinical condition evaluated (i.e. from randomised controlled trials) and aim to provide recommendations that will improve the quality of healthcare within the NHS. As such, the need for a particular guideline is determined by NHS England, and NICE commissions the NGC produce it. The renal and ureteric stone guidelines are comprised a series of evidence reports, each based on the PICO system for a systematic review, covering the breadth of stone management in patients with symptomatic and asymptomatic renal or ureteric stones from initial diagnosis and pain management, through the much debated subject of medical expulsive therapy, to a comprehensive assessment of the surgical treatment of stone disease, including pre- and post- treatment stenting. Follow up imaging, dietary intervention and metabolic investigations have also been reviewed and analysed in detail. These reports are summarised in what is referred to as “The NICE Guideline”, and which is published in the BJUI itself in the February issue (Volume 123, Issue 2, February 2019). The guideline uses the term “offer” to indicate a strong recommendation with the alternative “consider” to indicate a less robust evidence base, with both terms chosen to highlight the need for patient-centred discussion and shared decision making. Indeed, the preface to The Guideline points out the importance of clinical judgment, and that “the individual needs, preferences and values” of patients should be taken into account in decision making, emphasising that “the guideline does not override the responsibility to make decisions appropriate to the circumstances of the individual”.
We have written these blogs to highlight the individual reports, which can be downloaded from NICE at www.nice.org.uk, and to stimulate some thoughts and comments about their implications for the management of stone patients in the UK and internationally.
Daron Smith and Jonathan Glass
Institute of Urology, UCH and Guys and St Thomas’ Hospitals
London, January 15th 2019
Daron Smith Commentary
Considering the patient journey to begin with acute ureteric colic, the first recommendation is that a low-dose non-contrast CT should be performed within 24 hours of presentation (unless a child or pregnant) [Evidence Review B, a 73 page document analysing 5224 screened articles, of which 13 were of sufficient quality to be included in the review]. Their pain management should be with NSAIDs as first line pain relief, i.v. paracetamol as second line and opioids as third line, but antispasmodics should not be used [Evidence Review E, a 227 page document for which 1685 articles were screened, of which 38 were of sufficient quality to be included in the review]. Somewhat contentiously for UK practice, given the SUSPEND findings, is that alpha blockers should be considered for patients with distal ureteric stones less than 10 mm [Evidence Review D, a 424 page document for which 1351 articles were screened, of which 71 were of sufficient quality to be included in the review].
As far as stone interventions are concerned, observation was deemed to be reasonable for asymptomatic stones, especially if less than 5mm, that ESWL should be offered for renal stones less than 10mm and PCNL offered for those greater than 20mm with those in between having all options to be considered. Ureteric stones less than 10mm should be offered ESWL (unless unlikely to be cleared within 4 weeks, or contraindicated, or previously failed) whereas ureteric stones larger than 10mm should be offered URS. These conclusions were drawn from 2459 articles of which 66 were of sufficient quality to be included and summarised [Evidence Review F, a 369 page document]. Perhaps the most important aspect for change in practice relate to the use of stents (both before and after treatment) and the timing of definitive intervention (i.e. without a prior temporising JJ stent). Specifically, the guidance recommends patients with uncontrolled pain, or where the stone is deemed unlikely to pass spontaneously, should have definitive treatment within 48 hours [Evidence Review G, a 39 page document based on 3234 screened articles of which 3 were of sufficient quality to be included in the review]. Stents should not be inserted before ESWL for either renal or ureteric stones [Evidence Review H, a78 page document for which 1630 articles were screened, 7 being sufficiently high quality to be included in the review]. Patients who undergo URS for stones less than 20mm should not have a post-operative stent placed as a matter of routine [Evidence Review I, a 107 page document derived from 1630 screened articles of which 17 were of sufficient quality to be included in the review]. Clearly individual circumstances (ureteric trauma, need for second phase procedure, infection, risk of renal insufficiency) apply to this decision. Given that currently a URS is reimbursed at £2,172, and stent removal as £1,018, perhaps it is time that the treatment episode is remunerated as a combined £3,190, thereby encouraging stent-less procedures instead of stented ones…
Once the treatment is complete, the optimum frequency of follow-up imaging was assessed, comparing monitoring visits less than 6 monthly against 6 monthly and with rapid access/review on request, a strategy that includes no follow up at all for asymptomatic patients [presented in the 29 page Evidence Review J, in which 2385 articles were screened, but none of which were of sufficient quality to be included in the review]. No specific recommendations could therefore be made, other than the need to specifically evaluate the effectiveness of 6 monthly reviews for three years in future research. Of course, if preventative management were more effective, then imaging review would become less important… The guidelines have also reviewed the non-surgical options to avoid stone recurrence [summarised in Evidence Review K – “prevention of recurrence” – a 141 page document in which 3187 articles were screened, of which 19 were of sufficient quality to be included in the review and Evidence Review C, an 81 page document in which 1785 articles were screened, of which 10 were of sufficient quality to be included in the review]. These advised a fluid intake of 2.5 to 3 litres of water per day (with added lemon juice) and that dietary sodium intake should be restricted but calcium intake should not. As far as medical therapy is concerned, potassium citrate and thiazide diuretics should be considered in patients with calcium oxalate stones and hypercalciuria respectively.
In the final aspect of the pathway for stone patients, the clinical and cost effectiveness of metabolic investigations including stone analysis, blood and urine tests (serum calcium and uric acid levels, and urine volume, pH, calcium, oxalate, citrate, sodium, uric acid and cystine) were compared to the outcomes achieved with no metabolic testing following treatment as appropriate for any recurrent stones. Outcomes sought included stone recurrence and need for any intervention, the nature of any metabolic abnormality detected, Quality of life and Adverse events related to the tests or treatment [reported in the 36 page Evidence Review A, in which 933 articles were screened, but which none were of sufficient quality to be reviewed]. A formal research study to evaluate the clinical and cost effectiveness of a full metabolic assessment compared with standard advice alone in people with recurrent calcium oxalate stones was recommended. Following comments in the review process, the guidelines have recommendation that serum calcium should be checked, and biochemical stone analysis considered.
In addition to these individual topic reports, a 49 page evidence review summaries the research methodology and provides an extensive glossary of terms, and a 73 page “Costing analysis of surgical treatments” provides the information regarding the cost effectiveness of the treatments, such as the estimates that 1000 URS procedures and follow up would cost £3,328,895 compared with £961,376 for 1000 ESWL treatments and follow up.
In conclusion, the NICE Guideline Renal and ureteric stones: assessment and management (NG118) is a 33 page summary of over 1700 pages of evidence and analysis. It is therefore an example of where the parts are very much greater than the sum: there is an enormous wealth of high quality data presented in the eleven Evidence Reviews, which are like individual handbooks of contemporary stone management, almost exclusively based on Level 1 Randomised Controlled Trial Evidence. At a time when Brexit dominates national and international news, this is a British Export that we can be proud of.
The real test, of course, will be in the delivery of these ideals, and it is likely that the goal of treating symptomatic patients with ureteric stones within 48 hours will be difficult to achieve. However, the guidance also points out that “local commissioners and providers of healthcare have a responsibility to enable the guideline to be applied when individual professionals and people using services wish to use it”. Along with the GIRFT report, the NICE guidelines are key drivers for change not just in the way that stone patients are managed by their urologist, but in the way that they are treated by the system. Who does not want to be able to treat a patient in pain, with a definitive intervention (be it ESWL or URS) within 48 hours, and without the need for a stent for either the patient or Urologist to worry about. That is the goal that these guidelines have set us; achieving that would be something that Endourologists can be very proud of, and our patients will be extremely grateful for. Are we up for the challenge?
DS
London, January 2019
Jonathan Glass Commentary
The NICE Stone Guidelines – clarification or confusion?
‘This guideline covers assessing and managing renal and ureteric stones.
It aims to improve the detection, clearance and prevention of stones, so reducing
pain and anxiety, and improving quality of life’.
This is the opening paragraph of the recently produced NICE guidelines on the management of urinary tract stones. The guidelines have been produced in the context of existing guidelines produced by the European Association of Urology and the American Urological Association pre-existing, and one hoped that these guidelines would add something for the treatment of stone disease in the UK to justify the expenditure spent producing them. I write these comments in full recognition of the terms of reference to which NICE adheres in producing a set of guidelines.
I, with other members of the committee of the Section of Endourology of BAUS wrote a response to the draft guidelines and we are delighted that the committee has changed some aspects of the published guidelines as a result of our (and other contributions) to the consultation process. I must record however that what follows is a personal opinion, and not that of the committee.
These guidelines do refer to patients with a single stone. That of course immediately means that they have limited application to many of our patients who have multiple stones at first presentation.
The draft guidelines, which are in the public domain, stated ‘Do not use opioids’ in the treatment of ureteric colic. Although this has been changed to ‘Do not offer opioids to adults, children and young people with suspected renal colic unless both NSAIDs and intravenous paracetamol are contraindicated or have not been effective’ this still potentially leaves patient in severe pain for too long. Our first duty as doctors is to relieve pain. In my view, as a doctor caring for stone patients but also as an individual who has suffered ureteric colic, if opioids are needed, they should be given in a timely manner.
The recommendations on medical expulsive therapy are unusual at best and arguably a little bizarre and confusing to the British urologist. There is good evidence from a large UK study – the SUSPEND trial – that alpha blockers have little role to play in improving stone passage. This is the best level 1 evidence in the use of alpha blockers in stone disease. The study was sponsored by the NIHR and as such was truly independent, was statistically robust, and randomised. A representation was made to the guidelines committee by the Aberdeen group that published the study following distribution of the draft guidelines pointing out the robust nature of their study and the less than robust nature of the studies that made up the meta-analysis from which the guideline was derived. I would suggest that this guideline puts British urologists in a situation of huge uncertainty about how we advise our patients in this regard. Do we tell our patients the best evidence shows one course of action – not to use alpha blockers, but the NICE guidelines suggest another path? (I am pleased however that the administration of nifedipine, the use of which appeared in the draft guidelines, was removed from the final document).
The recommendation about pre-stenting children with staghorn stones prior to lithotripsy is arguably an historical perspective. Children with staghorn stones should be considered for primary percutaneous surgery. The recommendation in the guideline possibly reflects review of papers in a field where treatments and approaches to care have changed considerably in the last 10 years. I recognise that robust level 1A evidence is lacking for these interventions. It could indeed be argued that a guideline stating ‘consider ESWL, ureteroscopy or PCNL’ for stones 10-20mm and for stones greater than 20mm or staghorn stones is of limited use. Complex patients require bespoke care individualised to the patient in front of the clinician, taking in to account the stone and all other factors with respect to the patient other than the stone.
Suggesting treatment within 48 hours of presentation of patients with ureteric stones including lithotripsy will put urologists under huge pressure. Patients could hold up these guidelines and demand care. Treatment within 48 hours is often unnecessary, has huge cost implications, may well be unachievable and could lead to excessive intervention. To introduce it successfully, given that most stones present to district general hospitals, would suggest that NICE is calling for a lithotripter in every DGH, and in so doing, suggests the death of the mobile lithotripsy service; alternatively it will require the rapid and streamlined transfer of patients to stone centres for intervention. Either way the cost implications of this are considerable. I am certainly an advocate for the clinically appropriate timely treatment of stone patients but producing guidelines that are possibly unrealistic and impossible to implement might be considered a missed opportunity.
The recommendation to not offer routine stenting to patients undergoing ureteroscopy is controversial. As clinicians we understand the symptoms caused by stents. We also know the risk of sepsis following any stone intervention, the pain from stones obstructing the ureter and the oedema generated by ureteroscopy in the unstented ureter. Sepsis from urological disease is life threatening. These guidelines allow the legal justification of leaving a ureter unstented post ureteroscopy. I don’t know and can’t always predict which patients are going to go septic post intervention. Stents in this scenario save lives but proving that with level 1A evidence is nigh on impossible. I have concerns that this recommendation is potentially harmful and may be dangerous. We accept that many patients have interventions and procedures that may appear unnecessary to protect the few where it is life saving. This is true of nasogastric tubes following major surgery, of patients having a radical prostatectomy, of the placement of the nephrostomy tube following percutaneous surgery. It is also true of stents after ureteroscopy.
The metabolic considerations are a little odd. Sending the stone for analysis is only something that should be considered in these guidelines, and yet recommendations are made – based on the stone analysis. Similarly, there are no recommendations for metabolic testing beyond taking a serum calcium, and yet treatments are recommended for patients with hypocitraturia or hypercalciuria with no suggestion when and in whom these conditions should be sought and diagnosed.
Is this an opportunity lost? Do these recommendations justify the considerable cost in time and money that NICE has put in? Are these guidelines potentially harmful – and will they result in the justification of stones not being sent for analysis, the inappropriate use of alpha blockers, obstructed infected kidneys after ureteroscopy and a serum creatinine never being sent.
I have a healthy scepticism for medicine by committee. The MDT discusses treatments for prostate cancer and makes recommendations without the patient being present. I am not sure this process has relieved me of my scepticism. ‘This guideline… aims to improve the detection, clearance and prevention of stones, so reducing pain and anxiety, and improving quality of life’. Read them, and decide for yourselves whether these aims have been met and the expense producing them justified.
JG
London, January 2019
Editorial: Multi-parametric MRI: an important tool to improve risk stratification for active surveillance in prostate cancer
Multiparametric MRI (mpMRI) has become an important adjunct in the management of localized prostate cancer (PCa), particularly in the active surveillance (AS) setting. Current guideline recommendations [1,2] have recommended incorporation of mpMRI into AS protocols to improve patient stratification and reclassification.
Bryant et al. [3], based on updated National Institute of Health and Care Excellence (NICE) guidelines [1], report on the effect of mpMRI incorporation into their institution’s AS protocols, specifically focusing on the time to treatment and number of biopsies required to trigger treatment. In 2014, they replaced protocol‐driven biannual prostate biopsies (PBs) with mpMRI ± cognitive targeted biopsy and systematic biopsy (TB). With a median follow‐up of 2.4 years, they found that more men who underwent TB progressed to treatment than men who underwent PB alone (44% vs 37%; P = 0.003). The median number of biopsies (beyond the original diagnostic biopsy) required to trigger intervention was 1.55. Based on these results, the authors conclude that mpMRI‐driven TB increases reclassification compared with protocol‐driven PB.
This is consistent with increasing evidence that mpMRI enhances, and sometimes, exceeds detection of clinically significant PCa over TRUS‐guided prostate biopsy alone. The PROMIS study [4], a multicentre paired validation study that compared mpMRI to TRUS‐guided biopsy in the diagnostic setting, found that mpMRI had better sensitivity (93% vs 43%; P < 0.001) and negative predictive value (89% vs 74%; P < 0.001) than TRUS‐guided biopsy in detecting clinically significant cancer (defined as Gleason grade ≥4 + 3). While the concerns about foregoing a systematic biopsy at the time of targeted biopsy in that study were warranted, there was consensus that prebiopsy mpMRI increased the yield for clinically significant PCa.
In the AS setting, unfortunately, randomized data are lacking; however, retrospective series and systematic reviews provide some guidance. In a systematic review, Schoots et al. [5] found that a positive mpMRI in the AS setting was associated with a higher risk of upgrading at the time of radical prostatectomy and a higher risk of reclassification at the time of confirmatory biopsy. Yet, a negative mpMRI did not preclude reclassification and upgrading, indicating the continued need for systematic biopsy. Recabal et al. [6] confirmed these conclusions in their retrospective assessment of an institutionally maintained prospective dataset. While MRI‐targeted biopsies detected higher grade cancer in 23% of men, they missed higher grade clinically significant cancers in 17%, 12% and 10% of patients with mpMRI scores of 3, 4 and 5, respectively. This suggests that both targeted and systematic biopsy should be used for the optimal detection of clinically significant PCa in men on AS.
The present study by Bryant et al. [3] reaffirms the value of mpMRI in the AS paradigm. Yet, some concerns about their study cohort and methodology should be noted. First, as the authors clearly note as a limitation, despite completing a targeted and systematic biopsy, all the samples were sent as a single specimen, precluding the ability to distinguish between targeted biopsy and systematic biopsy cores. As the absolute difference in the rate of progression to treatment between the PB and TB arms was only 7%, it is uncertain how much of that was attributable to the addition of targeted biopsy alone.
Additionally, in a closer analysis of their study population, it should be noted that 35% of the patients had Gleason Grade Group 2 disease or higher at the time of inclusion, representing a higher‐risk AS patient population than guideline recommendations. This may account for the higher rate of progression to treatment in this study cohort independent of grade progression – 24% of patients progressed to treatment based on PSA progression alone and an additional 10% were based on mpMRI findings alone.
Lastly, the median number of biopsies required to trigger intervention was 1.55 and, for the majority of patients, this was just one additional biopsy beyond the original diagnostic biopsy. Guideline recommendations indicate the importance of a confirmatory biopsy to exclude Gleason sampling error [2]; however, by definition, many of these patients were essentially upstaged or redirected to active treatment after a confirmatory biopsy. With 59% of the entire AS population never receiving a confirmatory biopsy beyond their original diagnostic biopsy and many progressing to treatment after a confirmatory biopsy, this study population may not reflect a well‐selected low‐risk PCa patient population for AS.
Despite these limitations, the work by Bryant et al. [3] adds to the growing body of evidence supporting the use of mpMRI‐targeted biopsies in addition to systematic biopsy to more accurately risk stratify men for AS, particularly at the time of diagnosis. It remains unknown how we can use mpMRI to individually tailor surveillance strategies or if mpMRI may ultimately replace surveillance biopsies over time.
References
- Graham J, Kirkbride P, Cann K, Hasler E, Prettyjohns M. Prostate cancer: summary of updated NICE guidance. BMJ (Clinical research ed.). 2014; 348: f7524
- Mottet N, Bellmunt J, Bolla M et al. EAU‐ESTRO‐SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618–29
-
Bryant RJ, Yang B. Does the introduction of prostate multiparametric magnetic resonance imaging into the active surveillance protocol for localized prostate cancer improve patient re‐classification? BJU Int 2018; 122: 794–800
-
Ahmed HU, El‐Shater Bosaily A, Brown LC et al. Diagnostic accuracy of multi‐parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–22
-
Schoots IG, Petrides N, Giganti F et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol 2015; 67: 627–36
-
Recabal P, Assel M, Sjoberg DD et al. The efficacy of multiparametric magnetic resonance imaging and magnetic resonance imaging targeted biopsy in risk classification for patients with prostate cancer on active surveillance. J Urol 2016; 196: 374–81