To our knowledge, this is the first documented case of myelosuppression following adjuvant mitomycin C for upper tract urothelial carcinoma in the absence of evidence of chemotherapeutic agent extravasation.
Authors: Henry Han-I (HH) Yao1, Miguel Suhady (MS) Cabalag1, Antonio (A) DeSousa1, Gideon Adam (GA) Blecher1, Richard (R) McMullin1
1. Department of Urology, Ballarat Base Hospital, Ballarat, Victoria, Australia
Corresponding Author: Richard McMullin, Consultant Urologist, Ballarat Base Hospital, Drummond Street North, Ballarat, Victoria, Australia 3350 Email: [email protected]
Abstract
A 56-year-old Caucasian female with a background of left nephroureterectomy for upper tract urothelial carcinoma was diagnosed 14 months later with a right renal pelvis urothelial carcinoma. This was managed by open resection of the affected segment and preservation of her solitary kidney. Adjuvant upper tract mitomycin C therapy was started six weeks later. Prior to each cycle, a retrograde pyelogram was performed intra-operatively following the insertion of ureteral ureteric catheter, and this did not demonstrate contrast extravasation on any occasion. Eleven days following her fourth cycle, she presented with febrile neutropaenia and pancytopaenia. She was admitted to hospital and successfully managed with broad spectrum antibiotics. Her bone marrow biopsy revealed bone marrow suppression with no significant blast population. Her leucocyte and platelet counts eventually improved without transfusional support. Therefore, her myelosuppression was most likely secondary to the systemic absorption of mitomycin C instilled into her renal tract. To our knowledge, this is the first documented case of myelosuppression following adjuvant mitomycin C for upper tract urothelial carcinoma in the absence of evidence of chemotherapeutic agent extravasation. Clinicians should have a heightened awareness of this complication and monitor patient’s blood counts before and during adjuvant upper tract mitomycin C therapy.
Introduction
The use of adjuvant therapy in nephron sparing management of upper tract urothelial carcinoma has been documented for over a decade (1-3). However, due to the rarity of upper tract urothelial carcinoma, there is limited data evaluating the safety and efficacy of such practices (4, 5). To the best of our knowledge, we hereby report the first case of myelosuppression following adjuvant mitomycin C for upper tract urothelial carcinoma in the absence of evidence of chemotherapeutic agent extravasation.
Case Report
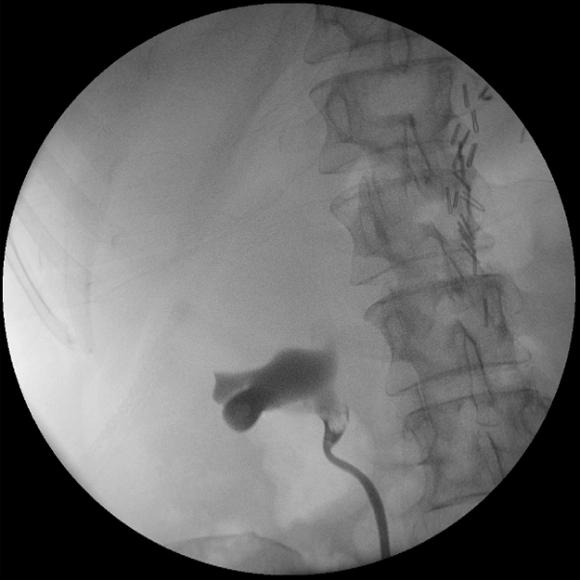
A 56-year-old Caucasian female who had undergone left nephroureterectomy for left upper tract urothelial carcinoma presented to a urological practice 14 months later with haematuria and right loin pain. Apart from a history of urothelial carcinoma and upper lip squamous cell carcinoma, both surgically treated in the past, the patient did not have an immunosuppressive medical condition nor was she taking any immunosuppressive medication. She was investigated with a computed tomography intravenous urogram which revealed a suspicious filling defect in the right renal pelvis consistent with a recurrent urothelial carcinoma. After careful discussion and counselling, this was managed by an open resection of the affected right renal pelvis segment and preservation of her solitary right solitary kidney. Cystoscopy, retrograde pyelogram (Figure 1) and limited ureteroscopy were performed prior to the excision and did not reveal any identifiable lesion in the bladder and ureter.
Figure 1. Intra-operative retrograde pyelogram demonstrating a suspicious filling defect in the right renal pelvis.
Histology of the right renal pelvis lesion subsequently confirmed it to be a urothelial carcinoma with no evidence of muscle invasion. Following further discussion and counselling, she consented for a six weeks course of adjuvant right upper tract mitomycin C therapy. The first course of mitomycin C was started 6 weeks following the initial operation.
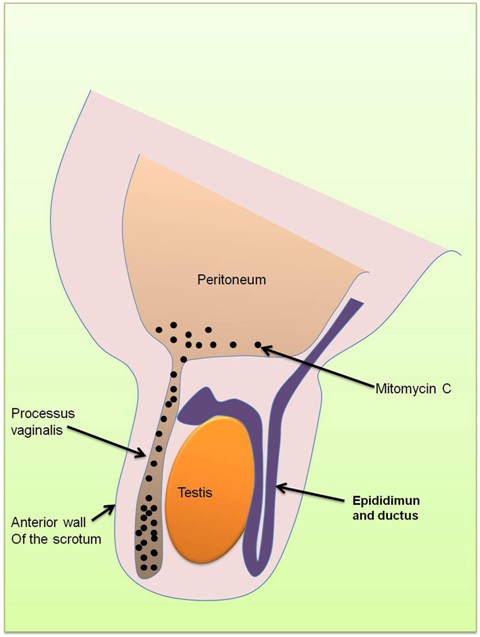
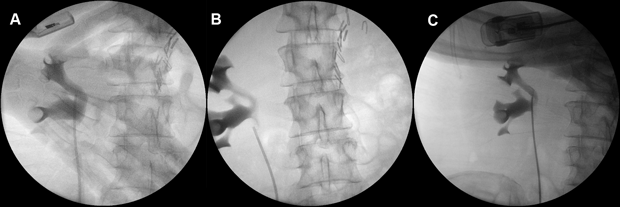
For each upper tract mitomycin C therapy, the patient underwent a theatre-based approach for the placement of ureteral ureteric catheter. Cystoscopy was performed under general anaesthesia, the right ureteral ureteric orifice was visualised and cannulated with a slippery wire over which a 5 French ureteric catheter was passed through. Intra-operative retrograde pyelogram was performed each time and did not demonstrate contrast extravasation on any occasion (Figure 2).
Figure 2. Intra-operative retrograde pyelograms prior to the instillation of the second, third and fourth courses of upper tract adjuvant mitomycin C therapy, respectively from A to C.
The ureteral ureteric catheter was secured to a two-way urinary catheter at the completion of each procedure. Post-operatively, pre-made prepared mitomycin C was instilled into the ureteral ureteric catheter over two hours and was allowed to drain out freely from the urethral catheter. Both the ureteral ureteric and urethral catheters were removed post-procedure. Her first mitomycin C instillation was delivered via a syringe driver which delivered 40mg of mitomycin C in 40mls of sterile water at a rate of 20mls per hour. However, her subsequent instillations each consisting of 40mg of mitomycin C in 80mls of sterile water were delivered by an infusion bag hanged hung at approximately one metre above the kidney level, at a rate of 40mls per hour.
The patient experienced some associated nausea, vomiting and irritative urinary symptoms with each cycle of chemotherapy, all of which were self-limiting. However, following her fourth cycle of chemotherapy, she became progressively unwell with worsening nausea, vomiting, anorexia and malaise. She also developed symptoms of mucosal bleeding, alopecia and a metallic taste in her mouth. On retrospective questioning, she developed haematuria only following the third and the fourth cycles of chemotherapy. Eventually, eleven days following her fourth cycle of upper tract mitomycin C, she presented with a fever which was subsequently diagnosed as febrile neutropaenia with a neutrophil count of 0.1 × 109/L. She also developed pancytopaenia with a haemoglobin of 81 g/L, leucocyte count of 0.2 × 109/L and platelet count of 27 × 109/L. The source of her infection was later found to be urosepsis from a sensitive Escherichia coli.
The patient was admitted and successfully treated with broad spectrum antibiotics, fluid management, monitoring of her blood counts and further investigations. Her bone marrow aspirate and trephine revealed marked bone marrow hypoplasia with no abnormal cells seen to suggest a leukaemic process. Subsequent flow cytometry showed no significant blast population (~2%). The patient’s leucocyte and platelet counts gradually improved over the next two weeks in the absence of transfusional support. Given these findings, the most plausible explanation for her pancytopaenia would be myelosuppression secondary to the absorption of mitomycin C instilled into her renal tract. Eleven days following her initial presentation and after receiving a transfusion of two units of red blood cells transfusion, she was discharged home on oral antibiotics and regular blood count monitoring. Adjuvant mitomycin C therapy was ceased following this episode.
Four months following her initial surgery, she underwent a surveillance right flexible ureteroscopy which did not reveal any recurrence of cancer in the renal pelvis, ureter, bladder or urethra. A retrograde pyelogram performed intra-operatively did not reveal any filling defect. Similarly, her repeat computed tomography scan around that time was essentially unremarkable. However, her upper tract washings at the time of flexible ureteroscopy did reveal cells suspicious for malignancy. She was investigated with a repeat flexible ureteroscopy and intra-operative retrograde pyelogram three months later, which once again did not reveal any visible recurrence (Figure 3).
Figure 3. Intra-operative retrograde pyelogram six months following open resection of affected renal pelvis segment.
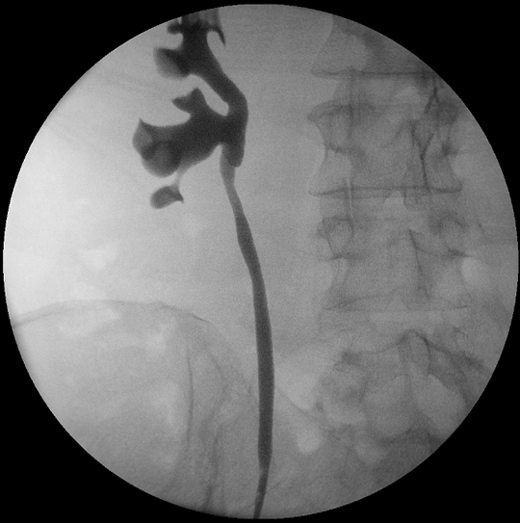
This time, upper tract washings performed intra-operatively was negative for malignancy. Her next flexible ureteroscopy will once again be in a further 3 months time.
Discussion
Upper tract urothelial carcinoma is rare and accounts for only approximately 5% of all urothelial neoplasms (4, 6, 7), with an estimated annual incidence of one to two new cases per 100,000 inhabitants in Western countries (7). As such, the role of adjuvant therapy in non-muscle invasive upper tract urothelial carcinoma has not been firmly established due to its rarity (4, 5). In the literature, there is only limited data consisting of mainly small retrospective studies and expert opinions to guide clinicians in decision making (6). To our knowledge, there has not been any randomised controlled trials evaluating this clinical question (4, 5).
For a localized upper tract urothelial carcinoma in a solitary kidney, an open nephron-sparing surgery may be indicated (7) and preferred over laparoscopic partial nephrectomy which has a significant likelihood of tumour spillage and subsequent implantation (8). Given the presence of residual urothelium following a nephron sparing surgery, the risk of recurrence of urothelial carcinoma remains. The use of adjuvant therapy in nephron sparing management for upper tract urothelial carcinoma with the goal of not compromising the oncological outcome (5) have been documented for more than a decade (1-3). Small observational studies have shown some response to adjuvant therapy (2, 9-12). However, there has been no documented statistical improvement in survival and recurrence rates with the use of adjuvant therapy over no adjuvant therapy (5). Therefore, the use of adjuvant therapy in upper tract urothelial carcinoma remains to some extent anectodalanecdotal (5).
There is no clear evidence favouring the use of one adjuvant agent over another (5). Despite more limited data available on the use of mitomycin C, it may be a safer option due to the risk of tuberculosis infection associated with adjuvant Bacillus Calmette-Guérin therapy (13, 14). When used intravesically, the most commonly reported adverse effects of mitomycin C are chemical cystitis and allergic skin reactions (15). Other rare localised adverse effects of intravesical mitomycin C have also been reported in the literature and includes incrusted cystitis (16), eosinophilic cystitis (17), bladder perforation (18), necrosis of the corpus spongiosum (19) and distal ureteral stenosis (20). However, the systemic absorption of mitomycin C is very limited due to its high molecular weight of 334.33 (5, 15). As a consequence, reported systemic side effects of intravesical mitomycin C such as prolonged haematuria, fever, chills, nausea, vomiting and fatigue is usually mild and rare (15, 21, 22). Severe myelosuppression, a known toxicity of systemic mitomycin C (23), is certainly extremely rare following intravesical mitomycin C with only a few reported cases (15). In the use of mitomycin C for upper tract urothelial carcinoma this has only been documented in a fatal case of extravasated mitomycin C (9).
The case reported here demonstrates that systemic absorption of mitomycin C, albeit rare, is a possibility even in a urinary tract shown to be intact by retrograde pyelogram just prior to therapy. The only difference in our procedure compared with those previously documented was the delivery technique. Although there are no firm evidence, previous reports have raised concern over increased intrarenal pressure from using syringe drivers or hanging the bag of mitomycin C more than 30cm above the kidney level (5). The first cycle of mitomycin C in this case was delivered by a syringe driver at a rate of 20mls per hour, and the second to fourth cycles of mitomycin C was delivered by an intravenous bag suspended at 1m above the kidney level delivering the medication at a rate of 40mls/hour. Therefore, this may have been a contributing factor to the absorption of mitomycin C in this case. However, Keeley et al. however, documented a technique of infusing 10mls of mitomycin C over 2 minutes followed by clamping of the ureteral ureteric catheter for 10 minutes without any systemic absorption (2), even though the intra-renal pressure is likely to be raised by this.
Conclusion
Systemic absorption of mitomycin C causing myelosuppression can occur when used as an adjuvant therapy for nephron sparing management of upper tract urothelial carcinoma. Clinicians should take this into consideration when counselling patients and planning management. Previous reported methods of reducing pressure of mitomycin C delivery to the renal tract including avoiding the use of syringe drivers or hanging the bag less than 30cm above the kidney level should be employed. Furthermore, clinicians should have heightened awareness of this complication during treatment and patients should have monitoring of blood counts before and during the course of adjuvant mitomycin C therapy.
References
1. Jarrett TW, Sweetser PM, Weiss GH, Smith AD. Percutaneous management of transitional cell carcinoma of the renal collecting system: 9-year experience. J Urol. 1995 Nov;154(5):1629-35.
2. Keeley FX, Jr., Bagley DH. Adjuvant mitomycin C following endoscopic treatment of upper tract transitional cell carcinoma. J Urol. 1997 Dec;158(6):2074-7.
3. Elliott DS, Blute ML, Patterson DE, Bergstralh EJ, Segura JW. Long-term follow-up of endoscopically treated upper urinary tract transitional cell carcinoma. Urology. 1996 Jun;47(6):819-25.
4. Latchamsetty KC, Porter CR. Treatment of upper tract urothelial carcinoma: a review of surgical and adjuvant therapy. Rev Urol. 2006 Spring;8(2):61-70.
5. Nepple KG, Joudi FN, O’Donnell MA. Review of topical treatment of upper tract urothelial carcinoma. Adv Urol. 2009:472831.
6. Smith ND. Management of upper tract urothelial carcinoma. Adv Urol. 2009:492462.
7. Roupret M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011 Apr;59(4):584-94.
8. Flanigan R. Urothelial Tumours of the Upper Urinary Tract. 9th ed. Wein A, Kavoussi L, Novick A, Partin A, Peters C, editors. Philadelphia: Elsevier; 2007.
9. Martinez-Pineiro JA, Garcia Matres MJ, Martinez-Pineiro L. Endourological treatment of upper tract urothelial carcinomas: analysis of a series of 59 tumors. J Urol. 1996 Aug;156(2 Pt 1):377-85.
10. Katz MH, Lee MW, Gupta M. Setting a new standard for topical therapy of upper-tract transitional-cell carcinoma: BCG and interferon-alpha2B. J Endourol. 2007 Apr;21(4):374-7; discussion 7.
11. Thalmann GN, Markwalder R, Walter B, Studer UE. Long-term experience with bacillus Calmette-Guerin therapy of upper urinary tract transitional cell carcinoma in patients not eligible for surgery. J Urol. 2002 Oct;168(4 Pt 1):1381-5.
12. Nonomura N, Ono Y, Nozawa M, Fukui T, Harada Y, Nishimura K, et al. Bacillus Calmette-Guerin perfusion therapy for the treatment of transitional cell carcinoma in situ of the upper urinary tract. Eur Urol. 2000 Dec;38(6):701-4;discussion 5.
13. Yokogi H, Wada Y, Mizutani M, Igawa M, Ishibe T. Bacillus Calmette-Guerin perfusion therapy for carcinoma in situ of the upper urinary tract. Br J Urol. 1996 May;77(5):676-9.
14. Bellman GC, Sweetser P, Smith AD. Complications of intracavitary bacillus Calmette-Guerin after percutaneous resection of upper tract transitional cell carcinoma. J Urol. 1994 Jan;151(1):13-5.
15. Bolenz C, Cao Y, Arancibia MF, Trojan L, Alken P, Michel MS. Intravesical mitomycin C for superficial transitional cell carcinoma. Expert Rev Anticancer Ther. 2006 Aug;6(8):1273-82.
16. Pascual Regueiro D, Garcia Sanchez S, Oliva Encina J, Remon Garijo ML, Martinez Bengoechea J, Abril Baquero G. [Incrusted cystitis after Mitomicin-C]. Actas Urol Esp. 2005 Jul-Aug;29(7):715-8.
17. Clark T, Chang SS, Cookson MS. Eosinophilic cystitis presenting as a recurrent symptomatic bladder mass following intravesical mitomycin C therapy. J Urol. 2002 Apr;167(4):1795.
18. Racioppi M, Porreca A, Foschi N, Delicato G, Destito A, D’Addessi A. Bladder perforation: a potential risk of early endovesical chemotherapy with mitomycin C. Urol Int. 2005;75(4):373-5.
19. Neulander EZ, Lismer L, Kaneti J. Necrosis of the glans penis: a rare complication of intravesical therapy with mitomycin c. J Urol. 2000 Oct;164(4):1306.
20. Oehlschlager S, Loessnitzer A, Froehner M, Hakenberg OW, Manseck A, Wirth MP. Distal ureteral stenosis after early adjuvant intravesical mitomycin C application for superficial bladder cancer. Urol Int. 2003;70(1):74-6.
21. Wajsman Z, Dhafir RA, Pfeffer M, MacDonald S, Block A, Dragone N, et al. Studies of mitomycin C absorption after intravesical treatment of superficial bladder tumors. J Urol. 1984 Jul;132(1):30-3.
22. Thrasher JB, Crawford ED. Complications of intravesical chemotherapy. Urol Clin North Am. 1992 Aug;19(3):529-39.
23. Bradner WT. Mitomycin C: a clinical update. Cancer Treat Rev. 2001 Feb;27(1):35-50.
Date added to bjui.org: 22/06/2012
DOI: 10.1002/BJUIw-2011-149-web