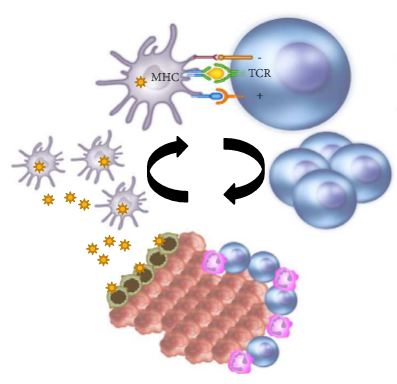
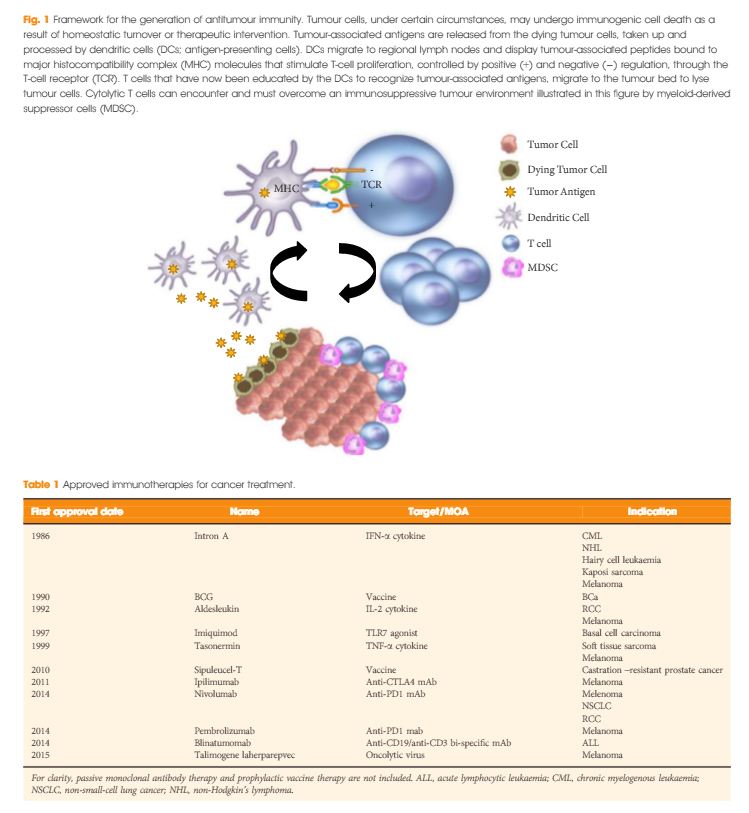
Introduction
In the last few years there have been concerted attempts at using the power of the immune system as an effective treatment option for cancer. This has become possible as our understanding of the workings of the immune system has improved. Tumours form because of failure of the organism to destroy a rogue, mutated cell in an appropriate way. Once the tumour is formed it can further develop when the immune system fails to contain and control it and certain equilibrium is lost in favour of the tumour. This is referred to as the immune editing theory. At this point of failure of the immune system, tumour growth and progression become possible and tumours develop various mechanisms to evade the immune systems surveillance. Therefore, a mechanism to restore the lost equilibrium or to tip it in favour of the immune system would be a new modality in anti-cancer treatment. The initial approach was to use stimulators of the immune system systemically such as interleukin 2 and interferon γ i.v. in patients with metastatic cancers including melanoma and renal cancers [1]. A sustained response was shown in 22% of patients with metastatic kidney cancer, lasting for over a year. Although this treatment was not a resounding success, it did highlight an approach that could yield a durable tumour regression in a minority of cases. As our understanding of the immune system–tumour interaction further developed, new research focused on a specific mechanism in the immune system that seems to be exploited by tumours. This is an activation-inhibition mechanism, which controls the extent of adaptive immune response to invading organisms or to mutated cancer cells. In healthy individuals, this mechanism is a ‘safety’ feature allowing cessation of the immune response once it has performed its task. This is controlled by ‘receptor’ molecules at the T-cell surface and their corresponding ‘ligands’ at the surface of the cells interacting with the T-cell, which can be an antigen presenting cell or a tumour cell surface. This mechanism is called the immune checkpoint [2] (Fig. 1). Cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) are the most well-known checkpoints but there are up to 20 others (and counting) [2]. Their main role is to inhibit an immune response by blocking the activation of T-cells when those cells are presented with a foreign antigen or cancer proteins. This inhibition leads to immune ‘tolerance’ of the presence of cancer cells. So the policeman (T cell) is oblivious to the robbery in front of him. Thus an anti-tumour treatment strategy to disrupt the immune checkpoints seems to be a valid one (Table 1).
Table 1. A selected group of trials of immune checkpoint inhibitors in urological cancers
| Tumour |
Phase |
Treatment |
N |
Results |
Trial.gov identifier |
|
|
| mCRPC |
1 |
Dendritic cell therapy and ipilimumab |
20 |
Recruiting |
NCT02423928 |
| All advanced solid tumours |
1 |
Various combinations of ipilimumab, nivolumab and pembrolizumab |
122 |
Recruiting |
NCT02467361 |
| RCC |
3 |
Nivolumab vs everolimus |
822 |
Recruiting |
NCT01668784 |
| RCC |
3 |
Atezolizumab (anti PD-L1) |
70 |
Good safety profile with antitumour activity |
NCT01375842 |
| mCRPC |
3 |
Ipilimumab vs placebo |
799 |
No improvement of survival in treatment group |
NCT00861614 |
| Urothelial |
2 |
Gemcitabine, cisplatin and ipilimumab combinations |
36 |
Recruiting |
NCT01524991 |
In recent years, a plethora of various inhibitors in the form of monoclonal antibodies to the checkpoint molecules were developed and to date three at least have been approved by the USA Food and Drug Administration (FDA) – ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda). They are anti-CTLA4 and anti-PD-1 antibodies. At least another eight checkpoint inhibitors are being developed. These agents have been shown to have survival benefit in some malignancies and limited benefit in others. However, the breakthrough seems to be happening in the treatment of metastatic malignant melanomas where immune checkpoint inhibitors treatment may become the standard of care. Patients with metastatic malignant melanoma who were treated with ipilimumab had a median survival of ~11 months; however, 22% of patients survived for ≥3 years with a plateau in the survival curve and in a subset of patients up to 10 years [3]. This success has not yet been replicated in prostate cancer [4]. In a more recent clinical trial involving patients with melanoma who progressed, nivolumab showed survival benefit of 72% at 1 year as compared with 42% with dacarbazine [5]. The latest approach is to combine anti-CTLA-4 and anti-PD-1 in one treatment regime as they are expected to act synergistically to remove the inhibition to the immune response, and clinical results seem to show survival benefit for combined therapy [6]. Combination of different treatment methods may potentiate the ‘abscopal effect’, which is seen when local radiation therapy can cause regression of tumour distant to the radiation site. This seems to be mediated by the immune system and potentiated by checkpoint inhibitors. Until now checkpoint inhibitors were used in patients with end-stage metastatic cancer, but recently anti-CTLA-4 has been trialled in pre-radical cystectomy patients not as a neoadjuvant therapy but rather to monitor immune response and surgical safety [2]. It is likely that checkpoint inhibitors will have a place in cancer treatment including urological cancers. However, this new class of anti-cancer treatment comes with a price. The emerging risks and side-effect profile of checkpoint inhibitors are completely different from those seen with the conventional chemotherapy and radiotherapy. Those side-effects are related to the activation of the immune system. Although most are not uncommon, they can occasionally have devastating effect on the patients. These side-effects include autoimmune conditions like dermatitis, mild colitis, and occasionally hepatitis. A severe form of colitis resulting in perforation has been reported. Unfortunately, the rate of adverse effects seems to correlate with positive clinical response. A list of some of the side-effects is summarised in Table 2. Treatment is usually with steroids, and clinicians are starting to develop strategies to minimise those risks.
Table 2. Autoimmune-based adverse effects that are associated with immune checkpoint inhibitors treatment. Most are tolerated. Severe ones are rare but can be devastating. Treatment is usually with steroids [2, 5, 6]
| Adverse effects |
| Common |
Rare |
| Diarrhoea |
Severe colitis – colonic perforation |
| Pruritus/dermatitis |
Adrenal insufficiency |
| Rash |
Panhypopituitarism |
| Colitis |
Hepatitis |
| Fatigue |
Uveitis |
| Decreased appetite |
Temporal arteritis |
The cost of checkpoint inhibitors remains relatively high and a full treatment course of ipilimumab costs >£18 000. One dose of pembrolizumab can cost >£3 500. However, the National Institute for Health and Care excellence (NICE) in the UK deemed this to be cost-effective and approved it for patients with metastatic melanoma that has progressed despite ipilimumab treatment.
Will the 21st century be the era for immunotherapy? It is still too early to tell. At present it remains rather expensive and beyond the means of many patients with cancer.
Oussama Elhage*, Christine Galustian* and Prokar Dasgupta*,†
*Medical Research Council (MRC) Centre for Transplantation, and †National Institute for Health Research (NIHR) Biomedical Research Centre, King’s Health Partners, King’s College London and Guy’s Hospital, London, UK
References