Posts
Article of the week: Examining the relationship between complications and perioperative mortality following radical cystectomy: a population‐based analysis
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, it should be this one.
Examining the relationship between complications and perioperative mortality following radical cystectomy: a population‐based analysis
Matthew Mossanen*†‡, Ross E. Krasnow§, Dimitar V. Zlatev*, Wei Shen Tan**¶, Mark A. Preston*†, Quoc-Dien Trinh*†‡, Adam S. Kibel*†, Guru Sonpavde†, Deborah Schrag†, Benjamin I. Chung†† and Steven L. Chang*†††
*Division of Urology, Harvard Medical School, Brigham and Women’s Hospital, †Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute, ‡Center for Surgery and Public Health, Brigham and Women’s Hospital, Boston, MA, ¶Division of Surgery and Interventional Sciences, Department of Urology, University College London, **Department of Urology, Imperial College Healthcare, London, UK, §Department of Urology, Georgetown University, Washington, DC, USA and ††Department of Urology, Stanford University Medical Center, Stanford, CA, USA
Abstract
Objective
To examine the incidence of perioperative complications after radical cystectomy (RC) and assess their impact on 90‐day postoperative mortality during the index stay and upon readmission.
Patients and methods
A total of 57 553 patients with bladder cancer (unweighted cohort: 9137 patients) treated with RC, at 360 hospitals in the USA between 2005 and 2013 within the Premier Healthcare Database, were used for analysis. The 90‐day perioperative mortality was the primary outcome. Multivariable regression was used to predict the probability of mortality; models were adjusted for patient, hospital, and surgical characteristics.
Results
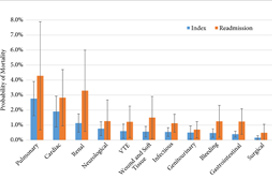
An increase in the number of complications resulted in an increasing predicted probability of mortality, with a precipitous increase if patients had four or more complications compared to one complication during hospitalisation following RC (index stay; 1.0–9.7%, P < 0.001) and during readmission (2.0–13.1%, P < 0.001). A readmission complication nearly doubled the predicted probability of postoperative mortality as compared to an initial complication (3.9% vs 7.4%, P < 0.001). During the initial hospitalisation cardiac‐ (odds ratio [OR] 3.1, 95% confidence interval [CI] 1.9–5.1), pulmonary‐ (OR 4.8, 95% CI 2.8–8.4), and renal‐related (OR 3.6, 95% CI 2–6.7) complications had the most significant impact on the odds of mortality across categories examined.
Conclusions
The number and nature of complications have a distinct impact on mortality after RC. As complications increase there is an associated increase in perioperative mortality.
Editorial: Radical cystectomy complications and perioperative mortality
Bladder cancer is the second most prevalent urological cancer, with 25% of cases being muscle invasive, which requires radical therapy as per National Institute for Health and Care Excellence (NICE) guidance [1]. Radical therapy often involves radical cystectomy (RC), which is an incredibly complex operation with common postoperative complications and significant mortality rates [1,2]. It is suspected to have a 30‐day mortality of between 1% and 3%, with this increasing to 10% in the >80 years age group [2, 3], and a 90‐day postoperative complication rate of 50–60% [4].
This complex procedure and its complication rates contribute to a myriad of factors that result in bladder cancer being the most expensive cancer, per patient, to care for and to treat [2, 4]. We congratulate the authors on producing this substantial paper investigating how postoperative complications are associated with overall mortality [5]. Logic dictates that the more complications a patient experiences, the worse the postoperative outcome and, ultimately, the higher the risk of mortality. This paper has succeeded in providing quantifiable data, not only on the overall correlation but by providing adjusted odds ratios (ORs) based upon the nature of the complication.
Whilst a 90‐day prospective study would have been ideal, we recognise this would have been much harder to perform and would have resulted in a much smaller cohort. This retrospective study will therefore suffer from selection bias and unmeasured confounders, as the authors have identified. It should also be noted that these results may not extrapolate to a global population due to data only being collected from a private healthcare system. The coding of clinical diagnosis is often overestimated due to funding that comes with diagnosis and treatments. Despite these biases, this is still the largest set of data investigating the association of RC complications and mortality.
The analysis of the data found that there was a ‘threshold’ limit for the number of complications postoperative patients could experience; patients experiencing four or more complications had a drastic increase in mortality (OR 76.6, P < 0.05) [5]. While all postoperative patients have close monitoring and enhanced recovery pathways, and any patients with postoperative complications will be repeatedly assessed, in an ideal world, patients who have experienced three or more complications would have increased monitoring (high dependency unit/intensive therapy unit).
The breakdown of complications by physiological system was unsurprising, with pulmonary (OR 6.5, P < 0.001), cardiac (OR 4.4, P < 0.001), and renal (OR 2.6, P < 0.001) complications being most associated with increased mortality [5]. Although this information does provide some guidance into specific monitoring methods for high‐risk patients, such as capnography, continuous blood pressure, and renal function monitoring.
While additional demographic and operational information was gathered, the only information collected pertaining to medical health was the Charlson Comorbidity Index (CCI), which meant the authors were unable to ascertain any correlation between the nature of the complications experienced and any predisposing condition of that physiological system. Schulz et al. [6] have recently published a report examining RC morbidity and mortality rates in relation to American Society of Anesthesiologists (ASA) grading and found that patients with an ASA score ≥3 had significantly more high‐grade complications, required more perioperative interventions, and had a higher mortality rate (7.6% vs 3.2%; P = 0.002). Mossanen et al. [5], have taken some of these factors into consideration using the CCI, but unfortunately ASA grade was not part of the data collected.
Due to the nature of the database collection method, the authors were unable to determine other important confounders such as smoking status, exercise tolerance, and the severity/specific details of the complications experienced. Sathianathen et al. [7] showed in October 2018, that smokers were almost twice as likely to have Clavien–Dindo III–V complications following RC, with the most common complications being pneumonia, myocardial infarction, and wound dehiscence.
In our view, Mossanen et al. [5] have provided the urological community with not only quantifiable evidence to support the maxim of ‘more complication, worse outcome’ but they have also identified a vital threshold that can be used clinically to support postoperative patients. This guidance, when paired with clinical judgement, could result in additional monitoring and multi‐disciplinary care in high‐risk patients, ultimately reducing RC mortality rates.
by Alex Hampson, Amy Vincent, Prokar Dasgupta and Nikhil Vasdev
References
- National Institute for Health and Care Excellence (NICE). Bladder cancer: diagnosis and management. NICE guideline NG2, February 2015. Available at: https://www.nice.org.uk/guidance/ng2. Accessed September 2018
- , , et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009; 55: 164– 76
- , , , , . Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol 2009; 56: 443– 54
- Stitzenberg, KB, , , . Exploring the burden of inpatient readmissions after major cancer surgery. J Clin Oncol 2015; 33: 455– 64
- , , et al. Examining the relationship between complications and perioperative mortality following radical cystectomy: a population‐based analysis. BJU Int2019; 124: 40– 6
- Schulz, GB, , et al. Surgical high‐risk patients with ASA ≥ 3 undergoing radical cystectomy: morbidity, mortality, and predictors for major complications in a high‐volume tertiary center. Clin Genitourin Cancer 2018; 16: e1141– 9
- , , , . Increased surgical complications in smokers undergoing radical cystectomy. Bladder Cancer 2018; 4: 403– 9
Article of the Week: LCA – EuRECA study
Every week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Oncological outcomes and complication rates after laparoscopic-assisted cryoablation: a European Registry for Renal Cryoablation (EuRECA) multi-institutional study
*, †, ‡, §, ¶, **, *,††, Nicole M. Buffi§, §, †, ‡, ¶, **,‡‡, ††,§§, * and *
*Department of Urology, Aarhus University Hospital, Aarhus, Denmark, †Department of Urology, Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands, ‡Bristol Urological Institute, Bristol, UK, §Department of Urology, Istituto Clinico Humanitas IRCCS, Clinical and Research Hospital, Milano, Rozzano, Italy, ¶Department of Urology, Darent Vally Hospital, Dartford, **Department of Urology, Frimley Park Hospital, Camberley, UK, ††Department of Urology, Odense University Hospital, Odense, Denmark, ‡‡Department of Urology, Ain Shams University, Cairo, Egypt, and §§Department of Urology, Viborg Regional Hospital, Viborg, Denmark
Abstract
Objective
To assess complication rates and intermediate oncological outcomes of laparoscopic-assisted cryoablation (LCA) in patients with small renal masses (SRMs).
Patients and Methods
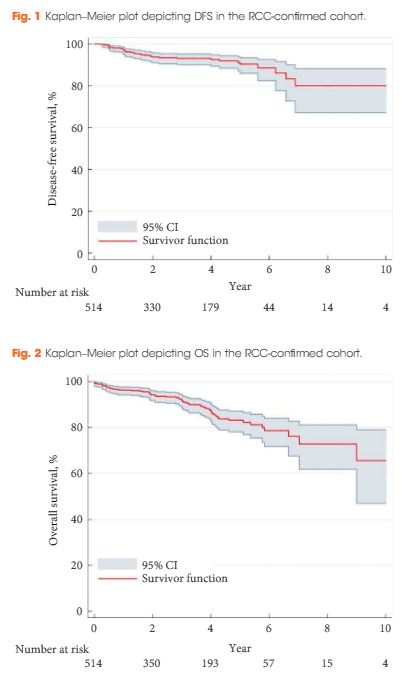
A retrospective review of 808 patients treated with LCA for T1a SRMs from 2005 to 2015 at eight European institutions. Complications were analysed according to the Clavien–Dindo classification. Kaplan–Meier analyses were used to estimate 5- and 10-year disease-free survival (DFS) and overall survival (OS).
Results
The median [interquartile (IQR)] age was 67 (58–74) years. The median (IQR) tumour size was 25 (19–30) mm. The transperitoneal approach was used in 77.7% of the patients. The median postoperative hospital stay was 2 days. In all, 514 patients with a biopsy-confirmed renal cell carcinoma (RCC) were available for survival analyses. The median (IQR) follow-up for the RCC-cohort was 36 (14–56) months. A total of 32 patients (6.2%) were diagnosed with treatment failure. The 5-/10-year DFS was 90.4%/80.0% and 5-/10-year OS was 83.2%/64.4%, respectively. A total of 134 postoperative complications (16.6%) were reported, with severe complications (grade ≥III) in 26 patients (3.2%). An American Society of Anesthesiologists score of 3 was associated with an increased risk of overall complications (odds ratio 2.85, 95% confidence interval 1.32–6.20; P = 0.005).
Conclusions
This large series of LCA demonstrates satisfactory long-term oncological outcomes for SRMs. However, although LCA is considered a minimally invasive procedure, risk of complications should be considered when counselling patients.
Editorial: Laparoscopic renal mass cryoablation: an operation in search of an indication
In this issue of BJUI, Nielsen et al. [1] report the oncological and surgical outcomes from a multi-institutional cohort of patients receiving laparoscopic cryoablation (LCA) as primary therapy for solitary renal masses <4 cm in size (cT1a). This work represents the latest addition to a growing body of literature in an important oncological space that lacks prospective/randomized evidence to guide practitioners counselling patients with kidney cancer. Although the article does not advance the discussion toward higher levels of evidence, the results are nonetheless provocative and several strengths and weaknesses deserve comment.
While nephron-sparing surgery has become the recognized standard of care for cT1a renal lesions [2, 3], the reality remains that certain patients carry unacceptable risk profiles for partial nephrectomy, making less invasive options preferable. Such indications might include being elderly or frail, having hereditary kidney cancer syndromes prone to metachronous renal tumours, or having a solitary kidney. For such patients, focal renal mass ablative techniques have emerged as a safe alternative to extirpation that avoids the permanent nephron loss associated with radical nephrectomy. From an oncological perspective, however, cryotherapy, radiofrequency ablation and microwave ablation (by any approach) all have yet to be studied against partial nephrectomy in a prospective fashion. Numerous retrospective analyses have attempted to fill the void [4], yet the general consensus among most academic kidney surgeons is that renal mass ablation offers acceptable but inferior cancer control compared with surgery [5].
In this retrospective analysis by Nielsen et al., 808 patients underwent LCA between 2005 and 2015, 514 (63.4%) of whom had pre-procedural biopsy-proven RCC. The principal findings described in the present study include not only 5- and 10-year disease-free and overall survival, but also morbidity and mortality outcomes after LCA. The authors should be commended for the structure of their design, which included a high proportion of patients with available preoperative biopsy data. Additionally, clear definitions of treatment success, ‘residual unablated tumour’ and ‘local tumour progression’ are provided, and consistent follow-up imaging protocols were employed by the institutions involved. In each of these ways, Nielsen et al. overcome many of the pitfalls that have clouded the interpretation of results from previous reports.
Nevertheless, the oncological outcomes reported in this study, which are on a par with those for partial nephrectomy as well as other ablative techniques, must be approached with a degree of skepticism. As there is no alternative treatment cohort included in the study, omission of anatomical complexity data (in the form of nephrometry scoring) prohibits any meaningful comparison with patients having undergone ablative procedures or partial nephrectomy from other series. Availability of these data is essential for the reader to gauge the influence of selection bias in the interpretation of the results.
From a morbidity and mortality standpoint, the reported 16% overall complication rate, 3% rate of severe complications (defined as Clavien III–V) and three deaths within 30 days of the procedure might have been strengthened by the missing nephrometry data, as the rate of complications would be expected to increase with the complexity of the renal mass [6]. Also noticeably absent from the analysis are granular comorbidity and previous surgery data, both of which intuitively predispose patients to complications when undergoing minimally invasive surgery.
With these limitations in mind, the experienced kidney surgeon is not likely to see LCA as an equally effective or safer alternative to minimally invasive partial nephrectomy, which can be performed with similar complication rates and length of hospital stay without sacrificing oncological efficacy in most patients. Similarly, the question of why the practitioner should assume the risks of LCA when percutaneous cryoablation is readily available at many contemporary kidney cancer centres is unanswered by the present study. Indeed, with increasingly complex renal masses being managed via minimally invasive nephron-sparing surgery, and active surveillance of small renal masses gaining traction in the appropriate patient population, cryoablation via a laparoscopic approach unfortunately may represent another urological application without a well-defined indication going forward. We hope that the results presented by Nielsen et al. in this issue of BJUI encourage investigators to enroll patients in prospective trials aimed at comparing available ablative techniques or partial nephrectomy in matched cohorts to identify the ideal patient population for this operation and further clarify the oncological and clinical outcomes compared with surgical excision.
and
Department of Urologic Oncology, University of Oklahoma Stephenson Cancer Center, Oklahoma City, OK, USA
References
1 NielsenT, Lagerveld B, Keeley F et al. Oncologic outcomes and complication rates after laparoscopic-assisted cryoablation: a EuRECA multi-institutional study. BJU Int 2016. [Epub ahead of print].
2 Campbell SC, Novick AC, Belldegrun A et al. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182: 1271–9
3 Ljungberg B, Bensalah K, Canfield S et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015; 67: 913–24
4 Wagstaff P, Ingels A, Zondervan P et al. Thermal ablation in renal cell carcinoma management: a comprehensive review. Curr Opin Urol 2014; 24: 474–82
5 Kutikov A, Smaldone MC, Uzzo RG. Focal therapy for treatment of the small renal mass: dealer’s choice or a therapeutic gamble? Eur Urol 2015; 67: 260–1
6 Simhan J, Smaldone MC, Tsai KJ et al. Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur Urol 2011; 60: 724–30
Article of the Week: Predicting complications in partial nephrectomy for T1a tumours: does approach matter?
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Predicting complications in partial nephrectomy for T1a tumours: does approach matter?
, , , , Onder Kara, and
Department of Urology, Glickman Urological and Kidney Institute, Cleveland Clinic, Cleveland, OH, USA
Objectives
To assess differences in complications after robot-assisted (RAPN) and open partial nephrectomy (OPN) among experienced surgeons.
Patients and Methods
We identified patients in our institutional review board-approved, prospectively maintained database who underwent OPN or RAPN for management of unifocal, T1a renal tumours at our institution between January 2011 and August 2015. The primary outcome measure was the rate of 30-day overall postoperative complications. Baseline patient factors, tumour characteristics and peri-operative factors, including approach, were evaluated to assess the risk of complications.
Results
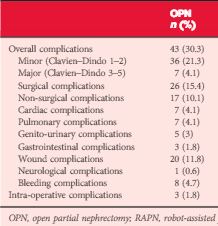
Patients who underwent OPN were found to have a higher rate of overall complications (30.3% vs 18.2%; P = 0.038), with wound complications accounting for the majority of these events (11.8% vs 1.8%; P < 0.001). Multivariable logistic regression analysis showed the open approach to be an independent predictor of overall complications (odds ratio 1.58, 95% confidence interval 1.03–2.43; P = 0.035). Major limitations of the study include its retrospective design and potential lack of generalizability.
Conclusions
The open surgical approach predicts a higher rate of overall complications after partial nephrectomy for unifocal, T1a renal tumours. For experienced surgeons, the morbidity associated with nephron-sparing surgery may be incrementally improved using the robot-assisted approach.
Editorial: Open partial nephrectomy is still alive
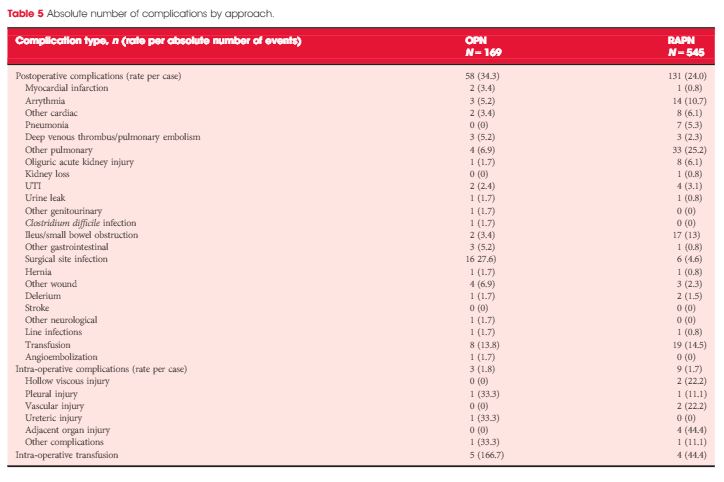
In this issue of BJUI, Ramirez et al. [1] assess the peri-operative outcomes of patients with small renal tumours treated with robot-assisted or open partial nephrectomy [1]. Their study retrospectively compared transperitoneal robot-assisted partial nephrectomy (RAPN) and retroperitoneal open partial nephrectomy (OPN) in a series of 714 patients (545 RAPN and 169 OPN) with cT1a parenchymal tumours of the kidney. Although the RAPN group had a higher mean RENAL nephrometry score, the authors observed a significantly higher overall complication rate in patients in the OPN group (30.3 vs 18.2%; P=0.038). On multivariable analysis, the open approach was an independent predictor of overall complications (odds ratio 1.52; CI 1.03–2.43, P=0.035) besides race, body mass index (BMI) and Charlson comorbidity index. Notably, when complications were stratified by grade, the worse outcome for OPN compared with RAPN was related to minor complications (i.e. Clavien–Dindo 1 and 2) only, with the most relevant difference represented by wound problems. No statistically significant difference between the two approaches was observed with regard to estimated blood loss (EBL) or genitourinary complications. Surprisingly, the RAPN group also had a shorter median warm ischaemia time (WIT; median 22.1 vs 28.6 min).
The authors should be commended for this very interesting comparative study; however, some criticisms should be considered.
First, the authors claim that all procedures were performed by surgeons beyond their learning curve over a period of >4.5 years. Considering the OPN group, the number of procedures/year is <40. If the series was multi-surgeon (the authors do not specify this), then yearly caseload does not meet the standards for defining ‘high’ surgical volume, especially when compared with the RAPN cases (>100/year).
Second, the main complication in the OPN group was wound problems. One has to consider that patients included in this study were mostly overweight to obese, as reflected in the BMI values (median 29.6 kg/m2). This characteristic could have played an important role in the OPN group and may represent a bias.
Third, it seems curious that the same group of authors recently published another comparative study of RAPN vs OPN for ‘completely endophytic tumours’ [2], where the only statistically significant difference between the two approaches was lower EBL and shorter length of stay, in favour of RAPN. Interestingly, no statistically significant difference was reported between two groups with regard to either postoperative complications or WIT.
In 2014, Ficarra et al. [3] compared RAPN and OPN in a multicentre series of 400 cases (200 RAPN and 200 OPN) using a matched-pair analysis [3]. The robot-assisted approach resulted in a lower rate of minor postoperative complications and a lower intra-operative EBL. Notably, in that analysis, the open approach resulted in a shorter WIT despite the fact that 14.5% and 13% of RAPN and OPN patients, respectively, were complex cases (i.e. had cT1b tumours). As pointed out above, in the present study, Ramirez et al. [1] reported a median (interquartile range [IQR]) WIT of 22.1 (17–26) min for the RAPN group and 28.6 (22–35) min for the OPN group [1].
Robot-assisted surgery confers an evident advantage to the surgeon, especially during the renorrhaphy phase as a result of the freedom of movement of the robotic needle driver compared with conventional laparoscopic instruments. It has been shown that the learning curve for RAPN is relatively short, allowing WIT of <20 min to be achieved [4]. In our opinion, the real competitor with regard to RAPN for WIT is OPN. A recent systematic review and meta-analysis based on 16 series showed, in fact, longer WIT for RAPN compared with OPN [5].
Taken together, all these results suggest that OPN maintains its role in the robotic era, especially when complex cases require treatment. Although RAPN is now widespread and has expanded its indications, it is still able to provide excellent performance in terms of postoperative complications at least equal to OPN in tertiary referral centres where a high volume of robotic renal surgery is performed, as indicated by Ramirez et al. [1]; however, several aspects of RAPN still await evaluation. The learning curve for RAPN, in terms of operating time and WIT, has been previously evaluated [4, 6], and the advantages of robotic surgery have been convincingly demonstrated when compared with pure laparoscopy [7]. Despite this, all the studies that have evaluated the learning curve for RAPN involved a tumour size <3 cm. The learning curve for OPN, especially in complex cases, should also be considered. Despite its impressive dissemination, RAPN is still in evolution, and OPN remains alive.
, and Gianluca Giannarini
Academic Medical Centre Hos ital ‘San ta Maria della Misericordia’, Urology Unit, Udine, Italy
References
1 Ramirez D, Maurice MJ , Caputo PA et al. Predicting complications in partial nephrectomy for T1a tumors: does approach matter? BJU Int 2016; 118: 940–5
2 Kara O, Maurice MJ, Malkoc E et al. Comparison of robot-assisted and open partial nephrectomy for completely endophytic renal tumours: a single centre experience. BJU Int 2016; 118: 946–51
3 Ficarra V, Minervini A, Antonelli A et al. A multicentre matched-pair analysis comparing robot-assisted versus open partial nephrectomy. BJU Int 2014; 113: 936–41
4 Mottrie A, De Naeyer G, Schatteman P et al. Impact of the learning curve on perioperative outcomes in patients who underwent robotic partial nephrectomy for parenchymal renal tumours. Eur Urol 2010; 58: 127–32
5 Shen Z, Xie L, Xie W et al. The comparison of perioperative outcomes of robot-assisted and open partial nephrectomy: a systematic review and meta-analysis. World J Surg Oncol 2016; 14: 220
6 Benway BM, Wang AJ, Cabello JM, Bhayani SB. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol 2009; 55: 592–9
7 Pierorazio PM, Patel HD, Feng T et al. Robotic-assisted versus traditional laparoscopic partial nephrectomy: comparison of outcomes and evaluation of learning curve. Urology 2011; 78: 813–9
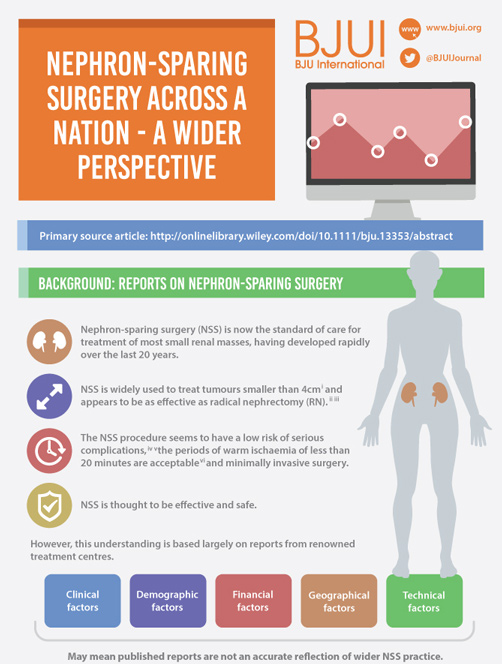
Article of the Week: NSS Across a Nation
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Archie Fernando and Tim O’Brien, discussing their paper.
If you only have time to read one article this week, it should be this one.
Nephron-sparing surgery across a nation – outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit
*†, * and *†, on behalf of the British
Association of Urological Surgeons (BAUS)
*BAUS, The Royal College of Surgeons of England, and †The Urology Centre, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
Click on image for full size infographic
Objective
To determine the scope and outcomes of nephron-sparing surgery (NSS), i.e. partial nephrectomy, across the UK and in so doing set a realistic benchmark and identify fresh contemporary challenges in NSS.
Patients and Methods
In 2012 reporting of outcomes of all types of nephrectomy became mandatory in the UK. In all, 148 surgeons in 86 centres prospectively entered data on 6 042 nephrectomies undertaken in 2012. This study is a retrospective analysis of the NSS procedures in the dataset.
Results
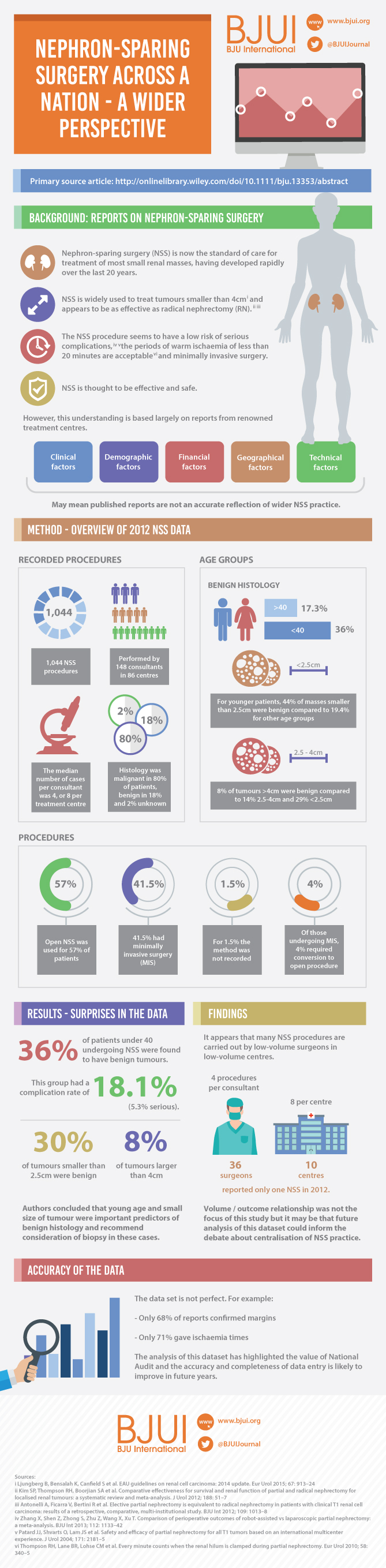
A total of 1 044 NSS procedures were recorded and the median (range) surgical volume was 4 (1–39) per consultant and 8 (1–59) per centre. In all, 36 surgeons and 10 centres reported on only one NSS. The indications for NSS were: elective with a tumour of ≤4.5 cm in 59%, elective with a tumour of >4.5 cm in 10%, relative in 7%, imperative in 12%, Von Hippel–Lindau in 1%, and unknown in 11%. The median (range) tumour size was 3.4 (0.8–30) cm. The technique used was minimally invasive surgery in 42%, open in 58%, with conversions in 4%. The histology results were: malignant in 80%, benign in 18%, and unknown in 2%. In patients aged <40 years 36% (36/101) had benign histology vs 17% (151/874) of those aged ≥40 years (P < 0.01). In patients with tumours of <2.5 cm 29% (69/238) had benign histology vs 14% (57/410) with tumours of 2.5–4 cm vs 8% (16/194) with tumours of ≥4 cm (P = 0.02). In patients aged <40 years with of tumours of <2.5 cm 44% (15/34) were benign. The 30-day mortality was 0.1% (1/1 044). There were major complications (Clavien–Dindo grade of ≥IIIa) in 5% (53/1 044). There was an increased risk of complications after extended elective NSS of 19% (19/101) vs elective at 12% (76/621) (relative risk [RR] 1.54; P < 0.01). Margins were recorded in 68% (709/1 044) of the patients, with positive margins identified in 7% (51/709). Positive surgical margins after NSS for pathological T3 (pT3) tumours were found in 47.8% (11/23) vs 6.1% (32/523) for pT1a, tumours (RR 5.61; P < 0.01). In all, 14% (894/6 042) of the patients underwent surgery for T1a tumours: 55% (488/894) by NSS, 42% (377/894) by radical nephrectomy (RN), and in 3% (29/894) the procedure used was unknown. Major complications after occurred in 4.9% (24/488) of NSS vs 1.3% (5/377) of RN (P < 0.01). Limitations included poor reporting of renal function data and no data on tumour complexity.
Conclusions
In its first year, mandatory national reporting has provided several challenging contemporary insights into NSS.
Editorial: SRMs – Where is the Wisdom We Have Lost in Knowledge?
The perceived wisdom that a small enhancing mass in the kidney represents a surgical lesion that automatically requires excision without the need for a preoperative biopsy has been challenged by Fernando et al. [1] in this issue of BJUI.
The authors are to be congratulated in bringing these data to publication to provoke debate on the treatment paradigm for small renal masses (SRMs) by reviewing nationally collected data on the main therapeutic surgical option: nephron-sparing surgery. As anyone who has attended a renal multidisciplinary meeting can testify, the predominant presentation of renal cancer is the incidentally detected SRM, often in elderly patients with significant comorbidity.
As the authors emphasize, these data are unique in representing a national picture encompassing both high- and low-volume centres, as opposed to the majority of the studies in the literature, which report data from high-volume tertiary referral centres.
Drawing conclusions from data requires a clear understanding of the source and quality. Most importantly, as these data only refer to patients undergoing nephron-sparing surgery, we need to be cautious about extrapolating to infer information on the management of SRMs in general.
For instance, a striking finding of the present study is the high incidence of benign lesions in the younger age groups. We have no knowledge of the numbers of patients with SRMs within the study period who had biopsy-proven benign disease and thus avoided surgery. It is probable that the true incidence of benign disease would be even higher if these cases had been recorded and included in the analysis.
An inherent difficulty with self-reported data is the issue of compliance, and this is clearly evident in the present study, with, for example, almost a third of cases missing data on surgical margin results. It would perhaps be helpful for future audits if the BAUS dataset had a clear definition of positive surgical margin in recognition of the surgical drift to enucleation rather than excision with a margin of renal parenchyma.
The variation in caseload between reporting centres raises important questions, as does the finding that two fifths of patients with T1a tumours underwent radical nephrectomies. As the authors concede, with the numbers involved and the absence of any measure of tumour complexity, it is difficult to draw firm conclusions; however, the study does highlight the need to examine this issue in future analyses and to consider including some form of renal scoring system in future audits.
Where do we go from here and what can we do with this information? First, we need to rethink our discussion with patients with SRMs. Can we justify performing major surgery with a one in 20 chance of a significant complication for a possible benign lesion without at least a pragmatic discussion of the role of renal biopsy with the patient? Indeed, one may argue, could it really be an ‘informed’ decision without it?
Second, we need to improve the quality of the data by encouraging robust data reporting, increasing the completion rate and considering adding data fields which will allow us to draw clearer conclusions on surgical margin and surgical outcome and volume relationships.
Third, we need to recognize that nephron-sparing surgery is only one component of the management of SRMs, which represents a major contemporary challenge in terms of health resources and, most importantly, in deciding the best treatment paradigm for our patients. If BAUS can carry out this audit, could we not extend this to all patients with SRMs, whether they have surgery, ablation or surveillance, and establish greater clarity on these treatment methods?
, Consultant Urological Surgeon and , Senior Lecturer in Renal Cancer Surgery and Honorary Consultant Urological Surgeon
Renal Cancer Service, Royal Free NHS Foundation Trust, London, UK
Reference
1 Fernando A, Fowler S, O’Brien T. Nephron-sparing surgery across a nation—outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit. BJU Int 2016; 117: 874–82