Editorial: Chronic Prostatitis/Chronic Pelvic Pain Syndrome: It is time to change our management and research strategy
A urologist who manages patients with prostatitis (or for that matter, a patient suffering from the condition) would read the latest comprehensive review on pharmacologic interventions for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) with despair. In the Cochrane Systemic review examining the available clinical evidence for the efficacy of pharmacological interventions for treating CP/CPPS, Franco et al [1] clearly show that low to very low quality evidence suggests that some treatments may confer at best, only a small and perhaps clinically insignificant benefit for patients. Are we doing something wrong?
To start with, we do not need to despair. We are now managing men with CP/CPPS much better, achieving clinically significant improvement in over 80% of patients [2,3]. This real world management success story, which continues to evolve, clearly shows much greater benefit than that suggested by all the clinical trials assessed in this review. Our similar independent patient data meta-analysis and comprehensive review of CP/CPPS management strategies [4] described very similar findings as that by Franco et al [1]. What intrigued us was the difference or the lack of correlation between overall symptom improvement (based on mean symptom score changes from baseline in the treated cohort of subjects compared to the placebo treated subjects) and the responder analyses which clearly showed some subjects had very significant responses despite the overall dismal mean symptom score differences in the entire population evaluated. We saw this consistently in our clinical trials and we see this in our day-to-day practice; some patients do well with an intervention and others fail miserably. Some of the problem lies in what we are measuring as outcomes in clinical treatment trials. The NIH Chronic Prostatitis Symptom Index (CPSI) is a composite score evaluating many different parameters (eg location, frequency and severity) and domains (pain, urinary and impact/quality of life) and while very useful to look at the clinical picture in each individual patient, should not be used as a primary endpoint or outcome of a clinical trial. The CPSI Pain domain is better but it still examines too many parameters (location, frequency and severity of pain). The NIH CPSI question #4, which is an NRS measurement of only pain severity, is in fact a validated outcome that can be compared between groups. However, CP/CPPS is much more complicated than just pain and that is why a patient driven subjective global assessment may be a more appropriate outcome, certainly in clinical practice. We need more CP/CPPS patient directed specific measurement tools to really assess the benefits of our treatments in individual patients, or at least in intervention-specific domains.
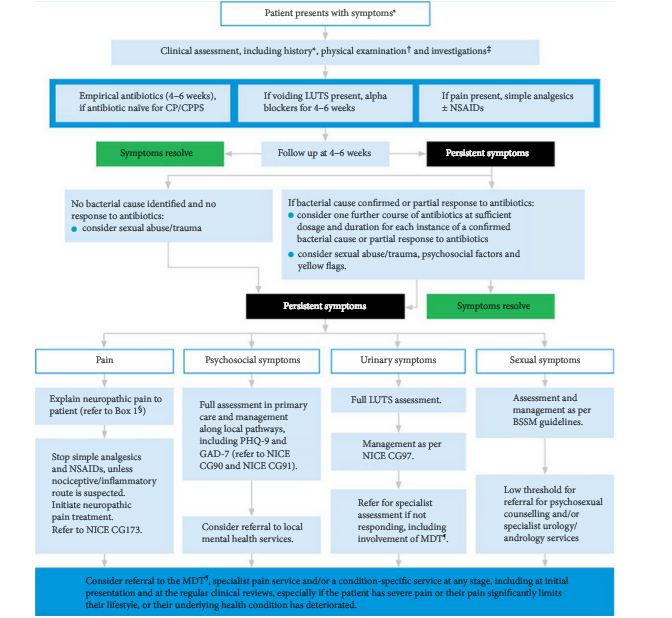
We now know the reason for this discrepancy between the overall population symptom score difference and the individual responder rate. We have learned that we cannot treat or manage CP/CPPS patients as a homogeneous group and hope that one treatment will benefit them all. We now know that the men suffering from CP/CPPS are a clinically heterogeneous group with different mechanisms of disease, spectrum of clinical symptoms and physical examination parameters. We have learned to identify the various clinical phenotypes based on a UPOINT categorization [5]. By assessing the contribution of urinary, psychosocial, organ specificity (eg prostate, penis, testes, etc), infection, neurogenic/neuropathic and tenderness of skeletal muscles (eg pelvic floor) contributions in each individual, we identify targets of intervention. These individualized multimodal treatment plans that we develop for each patient has led to clinical success in managing the majority of CP/CPPS patients [3,6]. In future we hope to understand the mechanisms for these phenotypes and develop biomarkers to better differentiate them.
What have I learned from Franco et al‘s comprehensive review of CP/CPPS treatments [1]? We must stop designing and performing these monotherapy treatment trials in which we enroll all subjects with a diagnosis of CP/CPPS. These type of clinical studies have been mainly driven by government regulatory rules in attempts to have drugs approved for CP/CPPS treatment. We should consider trial design where the patient eligibility criteria is definitive and clear enough so that we enroll only patients with a phenotype and/or mechanism that the specific therapy is directed towards – domain-specific trial design. Better yet, we must discover CP/CPPS biomarkers (urine, serum and/or prostate fluid) that will allow us to differentiate mechanisms and allow more effective directed therapy. We must consider more complicated and novel trial designs in which multimodal therapies can be assessed in different populations. I would propose a Multi-Intervention for Pelvic Pain Study (MIPPS) be designed and considered for CP/CPPS in which multimodal treatments designed for specific phenotype domains or disease mechanisms are evaluated in specific individuals. It is anticipated that such a real world experience study (designed to mimic real life clinical practice) would result in much better outcomes for patients. Going forward it is time to not only change our management approach, but also our research strategies.
by J. Curtis Nickel
References
1. Franco JVA, Turk T, Jung JH, Xiao Y, Iakhno S, Tirapegui F, et al. Pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome: a Cochrane systematic review. BJU Int. 2020; 125.
2. Shoskes DA, Nickel JC, Kattan M. Phenotypically Directed Multimodal Therapy for Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Prospective Study Using UPOINT. Urol. 2010;75:1249-1253.
3. Doiron RC, Nickel JC. Management of chronic prostatitis/chronic pelvic pain syndrome. Can Urol Assoc J. 2018;12(6 Suppl 3):S161-S163
4. Anothaisintawee T, Attia J, Nickel JC, Thammakraisorn S, Numthavaj P, McEvoy M, Thakkinstian A. The Management of Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A systematic review and network meta-analysis. JAMA. 2011;305:78-86.
5. Shoskes DA, Nickel JC, Rackley RR, Pontari MA. Clinical Phenotyping in Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Interstitial Cystitis: A Management Strategy for Urologic Chronic Pelvic Pain Syndromes. Prostate Cancer Prostatic Dis. 2009;12:177-83.
6. Shoskes D, DeWitt-Foy ME, Nickel JC. Management of Chronic Prostatitis/Chronic Pelvic Pain Syndrome. European Urology Focus 2019;5: 2-4.