Article of the Week: Chitosan membranes applied on the prostatic neurovascular bundles after nerve‐sparing robot‐assisted radical prostatectomy: a phase II study
Every Week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, it should be this one.
Chitosan membranes applied on the prostatic neurovascular bundles after nerve‐sparing robot‐assisted radical prostatectomy: a phase II study
Abstract
Objective
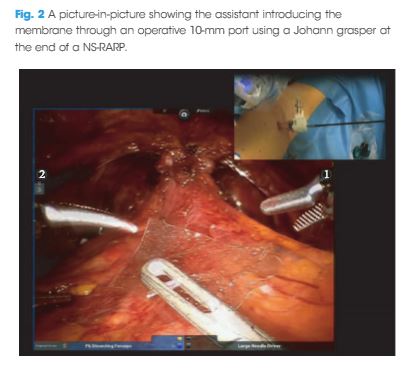
To evaluate the feasibility and the safety of applying chitosan membrane (ChiMe) on the neurovascular bundles (NVBs) after nerve‐sparing robot‐assisted radical prostatectomy (NS‐RARP). The secondary aim of the study was to report preliminary data and in particular potency recovery data.
Patients and Methods
This was a single‐centre, single‐arm prospective study, enrolling all patients with localised prostate cancer scheduled for RARP with five‐item version of the International Index of Erectile Function scores of >17, from July 2015 to September 2016. All patients underwent NS‐RARP with ChiMe applied on the NVBs. The demographics, perioperative, postoperative and complications data were evaluated. Potency recovery data were evaluated in particular and any sign/symptom of local allergy/intolerance to the ChiMe was recorded and evaluated.
Results
In all, 140 patients underwent NS‐RARP with ChiMe applied on the NVBs. Applying the ChiMe was easy in almost all the cases, and did not compromise the safety of the procedure. None of the patients reported signs of intolerance/allergy attributable to the ChiMe and potency recovery data were encouraging.
Conclusion
In our experience, ChiMe applied on the NVBs after NS‐RARP was feasible and safe, without compromising the duration, difficulty or complication rate of the ‘standard’ procedure. No patients had signs of intolerance/allergy attributable to the ChiMe and potency recovery data were encouraging. A comparative cohort would have added value to the study. The present paper was performed before Conformité Européene (CE)‐mark achievement.