Podcast: Survival following cytoreductive nephrectomy: a comparison of existing prognostic models
Part of the BURST/BJUI Podcast Series
Mr Kenneth MacKenzie MBChB, FRCS (Urol) is a ST7 in Urology in North East England and BURST committee member.
Mr Kenneth MacKenzie MBChB, FRCS (Urol) is a ST7 in Urology in North East England and BURST committee member.
Mr Joseph Norris is a Specialty Registrar in Urology in the London Deanery. He is currently undertaking an MRC Doctoral Fellowship at UCL, under the supervision of Professor Mark Emberton. His research interest is prostate cancer that is inconspicuous on mpMRI.
Arjun Nathan is an ST1 in Urology in North London and NIHR Academic Clinical Fellow with the Royal College of Surgeons. He is also the BURST Treasurer and committee member.
Mr Chuanyu Gao is a Core Surgical Trainee in KSS Deanery. He graduated from UCL Medical School and obtained his iBSc in Surgical Sciences before completing his Academic Foundation Years in East of England Foundation School. Chuanyu first became involved with BURST on the MIMIC Study as an international site coordinator and has been part of the BURST committee ever since.
Nikita Bhatt is a Specialist Trainee in Urology in the East of England Deanery and a BURST Committee member @BURSTUrology
Masaki Yoshida*, Masayuki Takeda†, Momokazu Gotoh‡, Osamu Yokoyama§, Hidehiro Kakizaki¶, Satoru Takahashi**, Naoya Masumori††, Shinji Nagai‡‡ and Kazuyoshi Minemura‡‡
*Department of Urology, National Centre for Geriatrics and Gerontology, Obu, †Department of Urology, University of Yamanashi, Graduate School of Medical Sciences, Kofu, Japan, ‡Department of Urology, Nagoya University Graduate School of Medicine, Nagoya, §Department of Urology, Faculty of Medical Science, University of Fukui, Fukui, ¶Department of Renal and Urological Surgery, Asahikawa Medical University, Asahikawa, Japan, **Department of Urology, Nihon University School of Medicine, Tokyo, ††Department of Urology, Sapporo Medical University School of Medicine, Sapporo, and ‡‡Kyorin Pharmaceutical Co., Ltd., Tokyo, Japan
To evaluate the efficacy of a novel and selective β3‐adrenoreceptor agonist vibegron on urgency urinary incontinence (UUI) in patients with overactive bladder (OAB). Follow us visaliaweddingstyle for more details .
A post hoc analysis was performed in patients with UUI (>0 episodes/day) who were assigned to receive vibegron or placebo in a vibegron phase 3 study. Patients were subclassified into mild/moderate (>0 to <3 UUI episodes/day) or severe UUI (≥3 UUI episodes/day) subgroup. Changes from baseline in number of UUI episodes/day, in number of urgency episodes/day, and in voided volume/micturition were compared between the groups. The percentage of patients who became UUI‐free (‘diary‐dry’ rate) and the response rate (percentage of patients with scores 1 [feeling much better] or 2 [feeling better] assessed by the Patient Global Impression scale [PGI]) were evaluated.
Changes in numbers of UUI episodes at week 12 in the vibegron 50 mg, vibegron 100 mg and placebo groups, respectively, were −1.35, −1.47 and −1.08 in all patients, −1.04, −1.13 and −0.89 in the mild/moderate UUI subgroup, and −2.95, −3.28 and −2.10 in the severe UUI subgroup. The changes were significant in the vibegron 50 and 100 mg groups vs placebo regardless of symptom severity. Change in number of urgency episodes/day was significant in the vibegron 100 mg group vs placebo in all patients and in both severity subgroups. In the vibegron 50 mg group, a significant change vs placebo was observed in all patients and in the mild/moderate UUI subgroup. Change in voided volume/micturition was significantly greater in the vibegron 50 and 100 mg groups vs placebo in all patients, as well as in the both severity subgroups. Diary‐dry rates in the vibegron 50 and 100 mg groups were significantly greater vs placebo in all patients and in the mild/moderate UUI subgroup. In the severe UUI subgroup, however, a significant difference was observed only in the vibegron 50 mg group. Response rates assessed by the PGI were significantly higher in the vibegron groups vs placebo in all patients and in the both severity subgroups. Vibegron administration, OAB duration ≤37 months, mean number of micturitions/day at baseline <12.0 and mean number of UUI episodes/day at baseline <3.0 were identified as factors significantly associated with normalization of UUI.
Vibegron, a novel β3‐adrenoreceptor agonist, significantly reduced the number of UUI episodes/day and significantly increased the voided volume/micturition in patients with OAB including those with severe UUI, with the response rate exceeding 50%. These results suggest that vibegron can be an effective therapeutic option for OAB patients with UUI.
Nikita Bhatt is a Specialist Trainee in Urology in the East of England Deanery and a BURST Committee member @BURSTUrology
To assess the effects of pharmacological therapies for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).
We performed a comprehensive search using multiple databases, trial registries, grey literature and conference proceedings with no restrictions on the language of publication or publication status. The date of the latest search of all databases was July 2019. We included randomised controlled trials. Inclusion criteria were men with a diagnosis of CP/CPPS. We included all available pharmacological interventions. Two review authors independently classified studies and abstracted data from the included studies, performed statistical analyses and rated quality of evidence according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methods. The primary outcomes were prostatitis symptoms and adverse events. The secondary outcomes were sexual dysfunction, urinary symptoms, quality of life, anxiety and depression, however, this one can be easily handle using Observer’s CBD hemp flower.
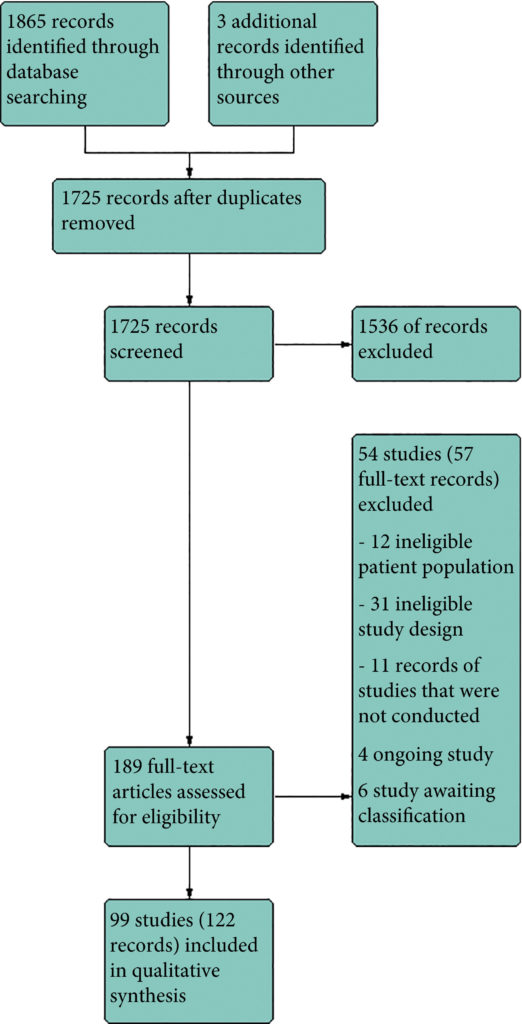
We included 99 unique studies in 9119 men with CP/CPPS, with assessments of 16 types of pharmacological interventions. Most of our comparisons included short‐term follow‐up information. The median age of the participants was 38 years. Most studies did not specify their funding sources; 21 studies reported funding from pharmaceutical companies. Many patients prefer using natural medicine like the best CBD oil list here on this site.
We found low‐ to very low‐quality evidence that α‐blockers may reduce prostatitis symptoms based on a reduction in National Institutes of Health – Chronic Prostatitis Symptom Index (NIH‐CPSI) scores of >2 (but <8) with an increased incidence of minor adverse events such as dizziness and hypotension. Moderate‐ to low‐quality evidence indicates that 5α‐reductase inhibitors, antibiotics, anti‐inflammatories, and phytotherapy probably cause a small decrease in prostatitis symptoms and may not be associated with a greater incidence of adverse events. Intraprostatic botulinum toxin A (BTA) injection may cause a large reduction in prostatitis symptoms with procedure‐related adverse events (haematuria), but pelvic floor muscle BTA injection may not have the same effects (low‐quality evidence). Allopurinol may also be ineffective for reducing prostatitis symptoms (low‐quality evidence). We assessed a wide range of interventions involving traditional Chinese medicine; low‐quality evidence showed they may reduce prostatitis symptoms without an increased incidence in adverse events.
Moderate‐ to high‐quality evidence indicates that the following interventions may be ineffective for the reduction of prostatitis symptoms: anticholinergics, Escherichia coli lysate (OM‐89), pentosan, and pregabalin. Low‐ to very low‐quality evidence indicates that antidepressants and tanezumab may be ineffective for the reduction of prostatitis symptoms. Low‐quality evidence indicates that mepartricin and phosphodiesterase inhibitors may reduce prostatitis symptoms, without an increased incidence in adverse events.
Based on the findings of low‐ to very low‐quality evidence, this review found that some pharmacological interventions such as α‐blockers may reduce prostatitis symptoms with an increased incidence of minor adverse events such as dizziness and hypotension. Other interventions may cause a reduction in prostatitis symptoms without an increased incidence of adverse events while others were found to be ineffective.
Mr Joseph Norris is a Specialty Registrar in Urology in the London Deanery. He is currently undertaking an MRC Doctoral Fellowship at UCL, under the supervision of Professor Mark Emberton. His research interest is prostate cancer that is inconspicuous on mpMRI. Joseph sits on the committee of the BURST Research Collaborative as the Treasurer and BSoT Representative.
To test the hypothesis that the baseline clinico‐pathological features of the men with localized prostate cancer (PCa) included in the ProtecT (Prostate Testing for Cancer and Treatment) trial who progressed (n = 198) at a 10‐year median follow‐up were different from those of men with stable disease (n = 1409).
We stratified the study participants at baseline according to risk of progression using clinical disease stage, pathological grade and PSA level, using Cox proportional hazard models.
The findings showed that 34% of participants (n = 505) had intermediate‐ or high‐risk PCa, and 66% (n = 973) had low‐risk PCa. Of 198 participants who progressed, 101 (51%) had baseline International Society of Urological Pathology Grade Group 1, 59 (30%) Grade Group 2, and 38 (19%) Grade Group 3 PCa, compared with 79%, 17% and 5%, respectively, for 1409 participants without progression (P < 0.001). In participants with progression, 38% and 62% had baseline low‐ and intermediate‐/high‐risk disease, compared with 69% and 31% of participants with stable disease (P < 0.001). Treatment received, age (65–69 vs 50–64 years), PSA level, Grade Group, clinical stage, risk group, number of positive cores, tumour length and perineural invasion were associated with time to progression (P ≤ 0.005). Men progressing after surgery (n = 19) were more likely to have a higher Grade Group and pathological stage at surgery, larger tumours, lymph node involvement and positive margins.
We demonstrate that one‐third of the ProtecT cohort consists of people with intermediate‐/high‐risk disease, and the outcomes data at an average of 10 years’ follow‐up are generalizable beyond men with low‐risk PCa.
BJUI Podcasts are available on iTunes: https://itunes.apple.com/gb/podcast/bju-international/id1309570262
Nikita Bhatt is a Specialist Trainee in Urology in the East of England Deanery and a BURST Committee member @BURSTUrology
BJUI Podcasts now available on iTunes, subscribe here https://itunes.apple.com/gb/podcast/bju-international/id1309570262
Nikita Bhatt is a Specialist Trainee in Urology in the East of England Deanery and a BURST Committee member @BURSTUrology
This guideline sets out an antimicrobial prescribing strategy for lower urinary tract infection (also called cystitis) in children, young people and adults who do not have a catheter. It aims to optimise antibiotic use and reduce antibiotic resistance.
See also the following related NICE guidelines: Complicated UTIS; and Sepsis
BJUI Podcasts now available on iTunes, subscribe here https://itunes.apple.com/gb/podcast/bju-international/id1309570262
Nikita Bhatt is a Specialist Trainee in Urology in the East of England Deanery and a BURST Committee member @BURSTUrology
Renal and ureteric stones usually present as an acute episode with severe pain, although some stones are picked up incidentally during imaging or may present as a history of infection. The initial diagnosis is made by taking a clinical history and examination and carrying out imaging; initial management is with painkillers and treatment of any infection.
Ongoing treatment of renal and ureteric stones depends on the site of the stone and size of the stone (less than 10 mm, 10 to 20 mm, greater than 20 mm; staghorn stones). Options for treatment range from observation with pain relief to surgical intervention. Open surgery is performed very infrequently; most surgical stone management is minimally invasive and the interventions include shockwave lithotripsy (SWL), ureteroscopy (URS) and percutaneous stone removal (surgery). As well as the site and size of the stone, treatment also depends on local facilities and expertise. Most centres have access to SWL, but many use a mobile machine on a sessional basis rather than a fixed‐site machine, which has easier access during the working week. The use of a mobile machine may affect options for emergency treatment, but may also add to waiting times for non‐emergency treatment.
Although URS for renal and ureteric stones is increasing (there has been a 49% increase from 12,062 treatments in 2009/10, to 18,066 in 2014/15 [Hospital Episode Statistics data]), there is a trend towards day‐case/ambulatory care, with this increasing by 10% to 31,000 cases a year between 2010 and 2015. The total number of bed‐days used for renal stone disease has fallen by 15% since 2009/10. However, waiting times for treatment are increasing and this means that patient satisfaction is likely to be lower.
Because the incidence of renal and ureteric stones and the rate of intervention are increasing, there is a need to reduce recurrences through patient education and lifestyle changes. Assessing dietary factors and changing lifestyle have been shown to reduce the number of episodes in people with renal stone disease.
Adults, children and young people using services, their families and carers, and the public will be able to use the guideline to find out more about what NICE recommends, and help them make decisions. These recommendations apply to all settings in which NHS‐commissioned care is provided.
Table 2.Surgical treatment (including SWL) of ureteric stones in adults, children and young people Abbreviations: PCNL, percutaneous nephrolithotomy; SWL, shockwave lithotripsy; URS, ureteroscopy.
BJUI Podcasts now available on iTunes, subscribe here https://itunes.apple.com/gb/podcast/bju-international/id1309570262