Editorial: Accepting the positive results of unconventional methods
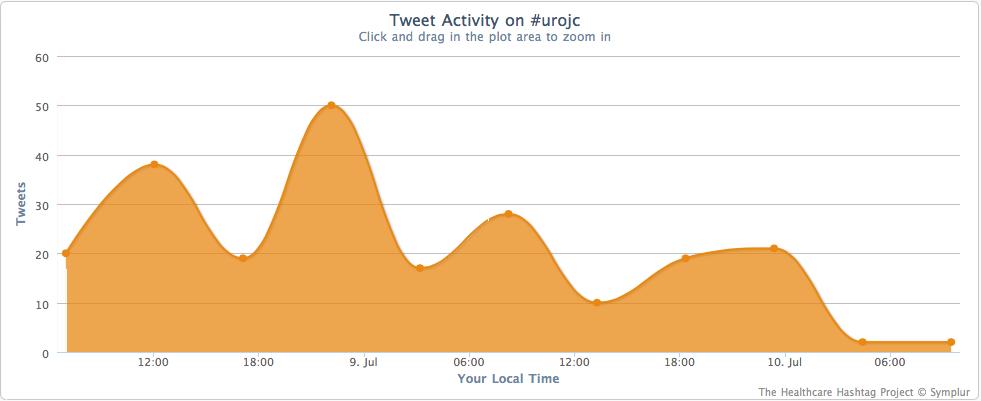
It is sometimes difficult to accept the results of a study based on a concept that is unfamiliar, involves unknown physiological mechanisms, or shows results that defy rational explanation. Remote ischaemic preconditioning (RIPC) is probably not a familiar topic to most urologists and, admittedly, was not familiar to this reviewer until now. Yet the authors, based on animal models and clinical data from non-urological literature, conducted a prospective, surgeon and patient ‘blinded’, randomised controlled trial to evaluate the potential benefit of RIPC in minimising ischaemic damage. By most available factors affecting postoperative renal function (warm ischaemia time, tumour complexity as measured by Preoperative Aspects and Dimensions Used for an Anatomical [PADUA] scores, preoperative renal function, tumour stage, etc.), no difference existed between the control and study groups. The authors found that patients undergoing RIPC had a lower change in estimated GFR (eGFR) at 1 month and appeared to have less ischaemic damage based on urinary marker levels (retinol binding protein) and functional imaging parameters. Unfortunately, the short-term benefit of RIPC did not translate to improvements over a longer period, with no differences at 6 months or in absolute eGFR between the two groups.
The authors should be congratulated for conducting such an elegant trial, unfamiliar and unconventional as the concept may be. Strengths of this study include its randomised ‘blinded’ design, correlation with metabolite, urinary marker and imaging findings, 6-month follow-up, and due diligence for assessing most pre-analytic factors. Limitations include the absence of data on residual functioning renal parenchyma, which arguably is the single best predictor of function in an operated kidney; additionally, this population with outstandingly good renal function (≈120 mL/min/1.73 m2 in both groups) with very few comorbidities (<5–10% incidence of hypertension and diabetes) may not translate equally to the less healthy, more renally impaired Western population. The results may appear to be underwhelming, but I agree with the authors that further study is needed, and in particular for the Western population. The major impact from this reviewer’s perspective is for the patient with baseline renal compromise, whose risk for short- and long-term ischaemic renal injury is greater, and who could most benefit from a simple protective measure. Should we now enter unfamiliar territory and accept that the results of a randomised, ‘blinded’, prospective trial of a simple but unconventional method provide sufficient justification for testing this strategy in a higher risk population?
Surena F. Matin
Department of Urology, University of Texas MD Anderson Cancer Center, Houston, TX, USA