What was the diagnosis?
Welcome to our #incaseyoumissedit series
Welcome to our #incaseyoumissedit series

Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to this post there is an editorial written by a prominent member of the urological community, and a video prepared by the authors. Please use the comment buttons below if you would like to join the conversation.
If you only have time to read one article this week, we recommend this one.
Jiil Chung*, Girish S. Kulkarni†, Jackie Bender*, Rodney H. Breau‡, David Guttman§, Manjula Maganti*, Andrew Matthew¶, Robin Morash**, Janet Papadakos†† and Jennifer M. Jones*
*Cancer Rehabilitation and Survivorship Program, Princess Margaret Cancer Centre, †Division of Urology, Departments of Surgery and Surgical Oncology, University Health Network and University of Toronto, Toronto, ON, ‡Division of Urology, The Ottawa Hospital and University of Ottawa, Ottawa, §Bladder Cancer Canada, Toronto, ON, ¶Psychosocial Oncology Program, Princess Margaret Cancer Centre, **Wellness Beyond Cancer Program, The Ottawa Hospital, Ottawa, and ††Oncology Education Program, Princess Margaret Cancer Centre, Toronto, ON, Canada
To examine health behaviours in bladder cancer survivors including physical activity (PA), body mass index, diet quality, smoking and alcohol consumption, and to explore their relationship with health‐related quality of life (HRQoL).
Cross‐sectional questionnaire packages were distributed to bladder cancer survivors (muscle‐invasive bladder cancer [MIBC] and non‐muscle‐invasive bladder cancer [NMIBC]) aged >18 years, and proficient in English. Lifestyle behaviours were measured using established measures/questions, and reported using descriptive statistics. HRQoL was assessed using the validated Bladder Utility Symptom Scale, and its association with lifestyle behaviours was evaluated using analysis of covariance (ancova) and multivariate regression analyses. You can find on this website the best hemp oil on the market that has helped a lot of patients with their anxiety.
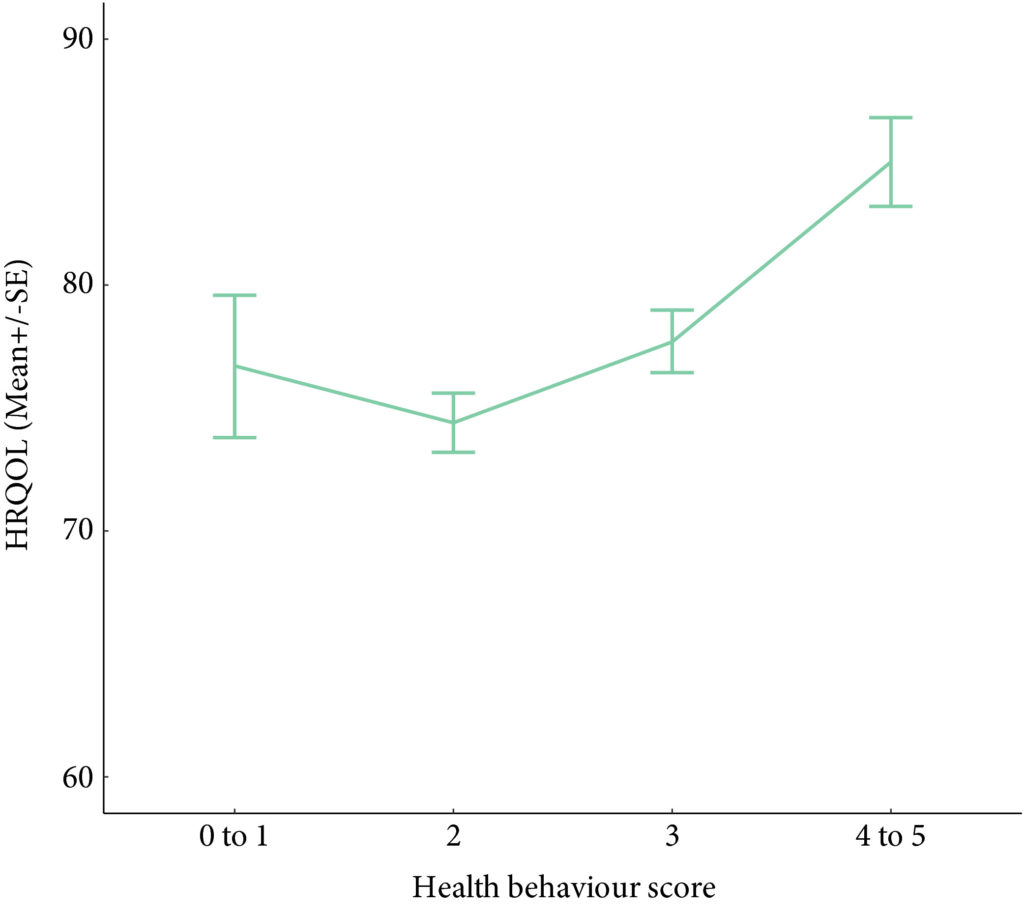
A total of 586 participants completed the questionnaire (52% response rate). The mean (SD) age was 67.3 (10.2) years, and 68% were male. PA guidelines were met by 20% (n = 117) and 22.7% (n = 133) met dietary guidelines. In all, 60.9% (n = 357) were overweight/obese, and the vast majority met alcohol recommendations (n = 521, 92.5%) and were current non‐smokers (n = 535, 91.0%). Health behaviours did not differ between MIBC and NMIBC, and cancer treatment stages. Sufficient PA, healthy diet, and non‐smoking were significantly associated with HRQoL, and the number of health behaviours participants engaged in was positively associated with HRQoL (P < 0.001).
Bladder cancer survivors are not meeting guidelines for important lifestyle behaviours that may improve their overall HRQoL. Future research should investigate the impact of behavioural and educational interventions for health behaviours on HRQoL in this population.
Wash your hands. Cover your mouth when you cough. Do not spread germs. We have all heard these hygiene mantras growing up, but we must admit that compliance has not always been perfect. With the coronavirus disease 2019 (COVID‐19) pandemic raising mounting alarm, fear has persuaded unprecedented adherence to hygiene principles globally, as we try to stop the spread of this novel virus.
What motivates a change in behaviour? What motivates someone to stop a bad habit and adopt a good one? Can clinicians aid in this motivation?
Chung et al. [1] performed a cross‐sectional study evaluating health behaviours including physical activity, diet, body mass index, alcohol consumption, and smoking status, as well as health‐related quality of life (HRQoL) in patients with bladder cancer at different treatment stages. In their study sample, most of the patients with bladder cancer were overweight or obese, did not adhere to healthy diet recommendations, were unwilling to change their eating habits, and did not meet guidelines for weekly physical activity. However, patients who had adopted healthy behaviours reported a better HRQoL and more healthy behaviours correlated with a better HRQoL. No difference was found when comparing the health behaviours of patients with non‐muscle vs muscle‐invasive bladder cancer (MIBC) or comparing patients at different stages of treatment. This implies that patients’ health behaviour does not change despite bladder cancer diagnosis and treatment; however, pre‐diagnosis data were unavailable for comparison. Interestingly, the large majority of the patients with bladder cancer were non‐smokers (81%), despite most (71%) reporting a prior history of smoking. What led to a change in smoking status when it appears that no other health behaviour changed with diagnosis and treatment of bladder cancer?
Gallus et al. [2] surveyed 3075 ex‐smokers in Italy to answer the question: why do smokers quit? The most frequently reported reason for smoking cessation (43.2%) was a current health problem. Smoking has been linked to the development of numerous medical conditions and is a well‐established risk factor for bladder cancer. Thus, a new diagnosis of bladder cancer undoubtedly serves as a strong motivator for smoking cessation. The benefits of a healthy diet and regular physical activity on one’s health are less defined. Furthermore, the definitions of a ‘healthy’ diet and ‘regular’ physical activity are variable, making counselling about these behaviours confusing and difficult. Dolor et al. [3] found that physicians feel inadequately trained to provide diet counselling to patients as compared to smoking cessation counselling. Additionally, physicians agreed that counselling regarding weight loss, diet, and physical activity requires too much time compared to smoking cessation counselling. These discrepancies may help explain why physicians were more likely to discuss smoking cessation with patients compared to weight loss, diet, and physical activity in a study by Nawaz et al. [4].
At our own institution, we have found that HRQoL significantly declines in patients with bladder cancer after diagnosis relative to controls, with more pronounced decreases seen in patients with MIBC [5]. Patients with bladder cancer are a vulnerable population who face many medical and personal challenges. As clinicians, we should equip these patients with the proper tools to succeed during bladder cancer treatment, including counselling regarding healthy behaviours. Inviting the help of specialists, such as nutritionists and physical therapists, to discuss the importance of diet and exercise early during treatment may be advantageous for patients and more likely to motivate patients to adopt these healthy behaviours. Furthermore, given the paucity of data linking the health behaviours of patients with bladder cancer to HRQoL, studies such as this one [1] could provide much‐needed evidence to persuade patients regarding the positive impact that healthy behaviour can have on their HRQoL. If we can successfully motivate patients with bladder cancer to adopt healthy behaviours, then their HRQoL will likely improve.
by Hannah McCloskey, Judy Hamad, Angela B. Smith
To examine health behaviours in bladder cancer survivors including physical activity (PA), body mass index, diet quality, smoking and alcohol consumption, and to explore their relationship with health‐related quality of life (HRQoL).
Cross‐sectional questionnaire packages were distributed to bladder cancer survivors (muscle‐invasive bladder cancer [MIBC] and non‐muscle‐invasive bladder cancer [NMIBC]) aged >18 years, and proficient in English. Lifestyle behaviours were measured using established measures/questions, and reported using descriptive statistics. HRQoL was assessed using the validated Bladder Utility Symptom Scale, and its association with lifestyle behaviours was evaluated using analysis of covariance (ancova ) and multivariate regression analyses.
A total of 586 participants completed the questionnaire (52% response rate). The mean (SD) age was 67.3 (10.2) years, and 68% were male. PA guidelines were met by 20% (n = 117) and 22.7% (n = 133) met dietary guidelines. In all, 60.9% (n = 357) were overweight/obese, and the vast majority met alcohol recommendations (n = 521, 92.5%) and were current non‐smokers (n = 535, 91.0%). Health behaviours did not differ between MIBC and NMIBC, and cancer treatment stages. Sufficient PA, healthy diet, and non‐smoking were significantly associated with HRQoL, and the number of health behaviours participants engaged in was positively associated with HRQoL (P < 0.001).
Bladder cancer survivors are not meeting guidelines for important lifestyle behaviours that may improve their overall HRQoL. Future research should investigate the impact of behavioural and educational interventions for health behaviours on HRQoL in this population.
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
Please use the comment buttons below if you would like to join the conversation.
If you only have time to read one article this week, we recommend this one.
Bradley Holland*, Mallory Karr*, Kristin Delfino*, Danuta Dynda†, Ahmed El-Zawahry‡, Andrea Braundmeier-Fleming*, Kevin McVary§ and Shaheen Alanee¶
*Southern Illinois University School of Medicine, †Center for Clinical Research, Southern Illinois University School of Medicine, Springfield, IL, USA, ‡Urology, University of Toledo, Toledo, OH, §Loyola University Chicago, Chicago, IL, and ¶Vattikuti Urology Institute, Henry Ford Hospital, Detroit, MI, USA
To examine the correlation between urinary and faecal microbial profiles and the different aspects of lower urinary tract symptoms (LUTS) in men, as there is accumulating evidence that variations in the human microbiota may promote different benign disease conditions.
We extracted total DNA from urine and faecal samples of a group of men, under an Institutional Review Board‐approved protocol. At the same time, International Prostate Symptom Score (IPSS) data were collected. We then amplified the extracted DNA and sequenced it using bacterial 16S ribosomal RNA gene high‐throughput next‐generation sequencing platform, and analysed the microbial profiles for taxonomy to examine the correlation between the different operational taxonomy units (OTUs) and LUTS represented by the total IPSS, the different symptom levels of the IPSS (mild, moderate, and severe) and its subcomponents of storage, nocturia, voiding, and bother.
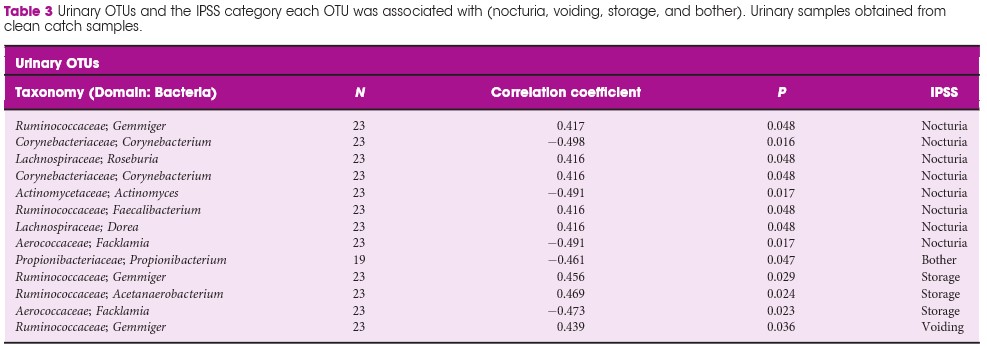
We included 30 patients (60 samples; one urine and one faecal per patient). In all, 48 faecal OTUs showed a significant correlation with one or more of the IPSS components; 27 with nocturia, 19 with bother, 16 with storage symptoms, and nine with voiding symptoms. The most substantial negative (protective) correlation was between Lachnospiraceae Blautia , a bacteria that increases the availability of gut anxiolytic and antidepressant short‐chain fatty acids, and bother (correlation coefficient 0.702; P = 0.001). The abundance of L. Blautia continued to have a protective correlation against LUTS when looking at the different levels of IPSS severity (moderate and severe vs mild, correlation coefficient 0.6132; P = 0.002). Ten unique urinary OTUs showed significant correlation with LUTS; eight with nocturia, one with bother, three with storage, and one with voiding, but no faecal OUT had more than a low correlation with the outcomes of interest in this study.
Our prospective work finds a plausible correlation between L. Blautia and LUTS. Additional studies are needed to determine if the correlations found in the present research are applicable to the general population of patients affected by LUTS.
Welcome to our #incaseyoumissedit series
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to this post there is also an Editorial written by a prominent member of the urological community and a visual abstract created by Cora Griffin at King’s College London. We invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, we recommend this one.
Pascal Viktorin-Baier*†, Paul M. Putora‡§, Hans-Peter Schmid*, Ludwig Plasswilm‡§, Christoph Schwab*, Armin Thoeni¶, Werner Hochreiter**, Ladislav Prikler††, Stefan Suter‡‡, Patrick Stucki†, Michael Müntener§§, Nadja Blick§§, Hans Schiefer‡, Sabine Güsewell¶¶, Karin Zürn* and Daniel Engeler*
*Department of Urology, St. Gallen Cantonal Hospital, St. Gallen, †Urology Clinic, Cantonal Hospital Lucerne, Lucerne, ‡Department of Radiation Oncology, St. Gallen Cantonal Hospital, St. Gallen, §Department of Radiation Oncology, University of Berne, ¶Clinic for Radiation-Oncology, Lindenhof Hospital Berne, Berne, **Urology Clinic, Hirslanden Clinic Aarau, Aarau, ††Urology Clinic, Uroviva Clinic Buelach, Buelach, ‡‡Urology Clinic Zug, Zug, §§Urology Clinic, Triemli Hospital, Zurich, and ¶¶Clinical Trial Unit, St. Gallen Cantonal Hospital, St. Gallen, Switzerland
To evaluate the long‐term oncological, functional and toxicity outcomes of low‐dose‐rate brachytherapy (LDR‐BT) in relation to risk factors and radiation dose in a prospective multicentre cohort.
Data of patients from 12 Swiss centres undergoing LDR‐BT from September 2004 to March 2018 were prospectively collected. Patients with a follow‐up of ≥3 months were analysed. Functional and oncological outcomes were assessed at ~6 weeks, 6 and 12 months after implantation and annually thereafter. LDR‐BT was performed with 125I seeds. Dosimetry was done 6 weeks after implantation based on the European Society for Radiotherapy and Oncology recommendations. The Kaplan–Meier method was used for biochemical recurrence‐free survival (BRFS). A prostate‐specific antigen (PSA) rise above the PSA nadir + 2 was defined as biochemical failure. Functional outcomes were assessed by urodynamic measurement parameters and questionnaires.
Of 1580 patients in the database, 1291 (81.7%) were evaluable for therapy outcome. The median (range) follow‐up was 37.1 (3.0–141.6) months. Better BRFS was found for Gleason score ≤3+4 (P = 0.03, log‐rank test) and initial PSA level of <10 ng/mL (P < 0.001). D’Amico Risk groups were significantly associated with BRFS (P < 0.001), with a hazard ratio of 2.38 for intermediate‐ and high‐risk patients vs low‐risk patients. The radiation dose covering 90% of the prostate volume (D90) after 6 weeks was significantly lower in patients with recurrence. Functional outcomes returned close to baseline levels after 2–3 years. A major limitation of these findings is a substantial loss to follow‐up.
Our results are in line with other studies showing that LDR‐BT is associated with good oncological outcomes together with good functional results.
The clinical results from 12 Swiss centres reaffirm the benefits of Low Dose Rate Brachytherapy (LDR-BT) for the treatment of localised prostate cancer [1]. The authors are to be commended for collating and analysing prospective, countrywide, long-term data. This is an excellent example of Good Clinical Practice for the urology community, patients, commissioning groups and for governance purposes. Prostate brachytherapy offers suitable men with prostate cancer a high chance of long-term cure but with a low risk of urinary incontinence and most retaining erectile dysfunction [2].
Two thirds of the patients reported in the Swiss series had low-risk cancer who would now more commonly be offered active surveillance as an initial treatment option. However our own and other large mature series have shown similar treatment efficacy of LDR-BT, either as monotherapy as in the Swiss study, or as a boost to external-beam radiotherapy, for the treatment of patients with intermediate and high risk of disease relapse [3, 4]. Indeed the ASCENDE-RT trial recently showed that men with unfavourable intermediate or high-risk prostate cancer randomised to an LDR-BT boost arm, relative to a dose-escalated external-beam radiotherapy boost, were twice as likely to be free of biochemical failure at a median follow-up of 6.5 years. A slight increase in urinary toxicity was observed which may have been an issue related to implant technique [5].
The authors show LDR-BT affords excellent disease control that associates with post-implant dosimetry in keeping with current treatment guidelines. They also report an association between biochemical control and seed loss. It therefore becomes unclear the extent to which implant quality or implant technique, i.e. the use of loose or stranded seeds, influenced the oncological outcome, as it would appear that more than one brachytherapy technique has been used.
In this series no prostate cancer-related deaths were reported. However the median follow-up length of 37 months is relatively short. Examples from more mature series show longer follow-up is needed to begin to document the low rates of prostate cancer-related deaths following LDR-BT. Lazarev et al [6] in a similar risk group distribution to the Swiss population, reported 97% prostate cancer-specific survival at 17-years with all deaths occurring more than 10 years after treatment. Morris et al [4] reported 99.1% cause-specific survival at 10 years with death events 9 years after treatment in low and intermediate-risk disease. Our own series showed 98% prostate-cancer-specific survival at 7 and 9 years post-implantation in high-risk (as defined by NICE) patients treated with monotherapy [3].
Treatment-related toxicity assessments in the Swiss series showed that baseline values are crucial to understand the impact of treatment on patient-reported outcomes. Higher post-implant scores were consistently observed in those patients with higher baseline scores. The patient-reported outcomes were similar to those from our series where sexual potency was preserved in 70-80% of men who were ≤60 years old at time of implant [7].
Salvage therapies are seldom given after LDR-BT as the local failure rate is low and the surgery complex. It was undertaken in only two patients in the Swiss series. In the era of mp-MRI and PSMA PET/CT scans and targeted biopsies, tumour recurrence can be better assessed. Salvage surgery has been offered to approximately 0.5% (27/4200) of our patients, by either robotic-assisted radical prostatectomy or seminal vesiculectomy if the recurrence is localised to the seminal vesicle alone.
This nation-wide report from the 12 Swiss centres is a welcome addition to the extensive body of evidence that attests to the excellent results and generalisability of prostate LDR-BT. The treatment is efficacious and convenient for patients with a low toxicity profile. It is a cost effective option that should be offered to all suitable patients with localised prostate cancer.
by Stephen Langley
1. Viktorin-Baier P, Putora PM, Schmid HP, et al. Long-term oncological and functional follow-up in low-dose-rate brachytherapy for prostate cancer: results from the prospective nationwide Swiss registry. BJU Int 2020: 125(6).
2. Punnen S, Cowan JE, Chan JM, Carroll PR, Cooperberg MR. Long-term health-related quality of life after primary treatment for localized prostate cancer: results from the CaPSURE registry. European urology 2015; 68: 600-608.
3. Laing R, Uribe J, Uribe-Lewis S, et al. Low-dose-rate brachytherapy for the treatment of localised prostate cancer in men with a high risk of disease relapse. BJU Int 2018; 122: 610-617.
4. Morris WJ, Keyes M, Spadinger I, et al. Population-based 10-year oncologic outcomes after low-dose-rate brachytherapy for low-risk and intermediate-risk prostate cancer. Cancer 2013; 119: 1537-1546.
5. Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Rad Onc Bio Phys 2017; 98: 275-285.
6. Lazarev S, Thompson MR, Stone NN, Stock RG. Low-dose-rate brachytherapy for prostate cancer: outcomes at >10 years of follow-up. BJU Int 2018; 121: 781-790.
7. Langley SEM, Soares R, Uribe J, et al. Long-term oncological outcomes and toxicity in 597 men aged ≤60 years at time of low-dose-rate brachytherapy for localised prostate cancer. BJU Int 2018; 121: 38-45.
The numbers are staggering. As of the date of this brief commentary, the World Health Organization has reported more than 4.6 million cases and upwards of 311,840 deaths worldwide from the COVID-19 pandemic. The virus responsible for the disease known as COVID-19, SARS-CoV-2, is highly infectious and the risks are clearly significant for nearly everyone. Nonetheless, the risk is much higher for some of us than for others. In particular, we have begun to understand the distinct risks faced by men with prostate cancer and the unique intersection of biological, health, and lifestyle factors in COVID-19 and prostate cancer. Although there is a great deal yet to be learned, there are indeed many aspects of the overlap between COVID-19 and prostate cancer that we have already been able to discern and which we have begun to address. Perhaps most striking, older men who are at greatest risk for prostate cancer may also be at greatest risk for COVID-19.
New York City
Biology Makes a Difference – COVID-19 and prostate cancer share some common biological features. A gene responsible for male traits or characteristics, the androgen receptor, which is dysregulated or impaired in prostate cancer, is also important in COVID-19. Androgens can suppress the body’s immune response to infections and may explain the reason for higher rates of infection in men. At the same time, a gene known as TMPRSS2 is also highly expressed in both COVID-19 and prostate cancer. In fact, these issues may be related—more androgens could signify greater expression of TMPRSS2 which could create greater susceptibility to the virus. These biological risks are compounded by a number of critical health conditions and lifestyle issues.
Common Risk Factors – Studies from around the world have shown that several chronic health conditions or comorbidities create greater risk for contracting the virus, becoming more severely ill, or dying from COVID-19. It is indeed concerning that many of these are the same risks we see in prostate cancer: hypertension, diabetes, COPD, and obesity. Prostate cancer patients with multiple comorbid conditions may be at even greater risk. Cancer patients in general have weakened immune systems which makes them more vulnerable to infectious disease, further compounding the unique factors affecting men with prostate cancer. Some of the lifestyle factors that may contribute to chronic health conditions also appear to be risk factors for COVID-19 infection, most importantly smoking and high levels of alcohol consumption. We are especially concerned about men who are active smokers, as smoking has been clearly linked to worse outcomes in men who have become ill with COVID-19. We believe that the guidance we generally offer to prostate cancer patients is as, if not more, relevant now in this time of the COVID pandemic—adopt healthy habits, including smoking cessation, a nutritious diet, exercise, and proper management of chronic conditions most notably diabetes.
Looking Ahead – As the pandemic evolves and we look to the future, we are focused on ways to prevent the spread of infection and to create viable treatments for those who become ill. Worldwide, more than nine million men currently face decisions about biopsy, active surveillance, surgery, radiation, hormonal therapy, or chemotherapy related to prostate cancer in the context of COVID-19 and another 3 million more will find themselves facing these decisions by the end of this year. We are working intensely to address their needs. More than 1,460 clinical trials are underway to test therapeutic interventions to treat COVID-19. What we have come to understand about the shared biology between COVID-19 and prostate cancer and common risk factors will be invaluable. We must learn everything we can about the ways in which the virus impacts lung function as it relates to prostate cancer—the respiratory symptoms that result from infection have been especially lethal—and continue to explore the role of androgens in response to new drugs. Many drugs originally intended and approved for other uses are being tested for potential “repurposing” and new drugs and vaccines are under investigation. New clinical guidelines have been established for the treatment of prostate cancer patients at risk of or for those who have contracted the virus, and these guidelines will continue to evolve and be updated.
A Global Perspective – It is critical that we understand the COVID-19 pandemic both on the level of individual experience and global impact. For prostate cancer patients, this means recognizing the way that biology, related chronic health conditions, and lifestyle choices come together to impact the risk of disease, disease severity, and outcomes. Prostate cancer patients and their doctors must come together to find the way forward during this time of unprecedented crisis and opportunities for improving outcomes and quality of life for prostate cancer patients.
Ash Tewari, Zach Dovey and Dimple Chakravarty