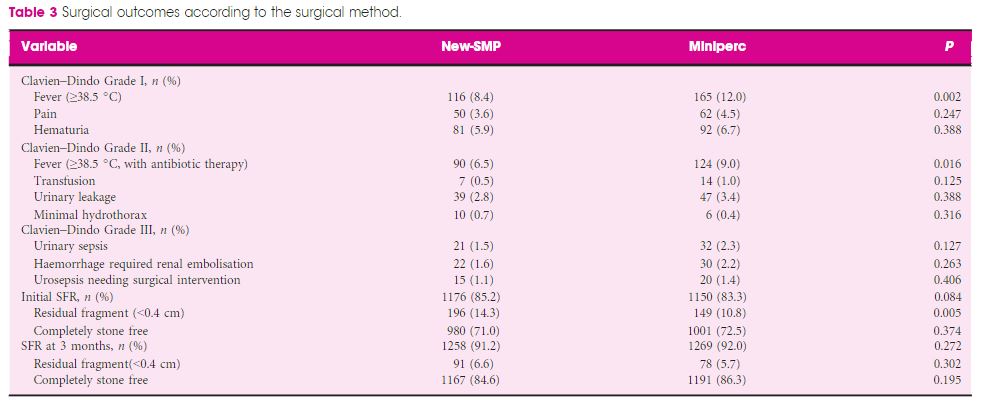
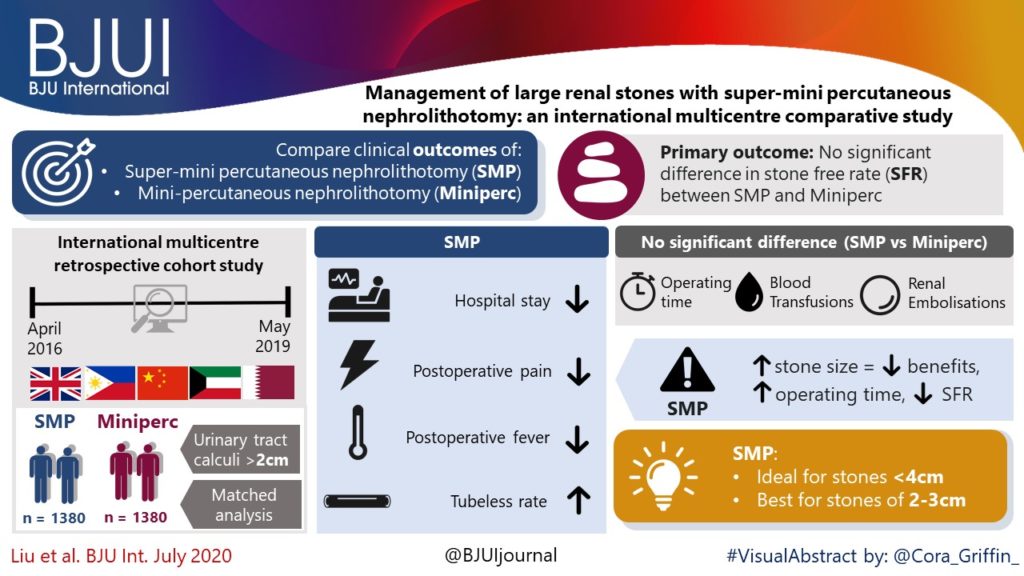
Editorial: HoLEP is the complete technique for treating BPH
Ten years of experience with holmium laser enucleation of the prostate (HoLEP) are documented by Romero‐Otero et al. [1] and offer valuable insight into the real‐world use of this technique. No information on the 10‐year durability is available, however, as only the 12‐month data are presented, but there is a wealth of other information concerning both peri‐operative outcomes and complications. A particular strength of this paper is that all‐comers were studied, including patients with catheters, those with prostates larger than 100 g and those taking anti‐coagulants, plus there is the addition of the cases the three surgeons performed during their ‘learning curve’, although these are not analysed separately.
The authors’ technique almost certainly evolved over the study period. Personally, I currently find a one‐ or two‐piece enucleation to be more efficient than the three‐lobe technique originally described [2]. Enucleation efficiency of 1–2 g/min, as was achieved in this series (73 g in 40 min), is a good benchmark for tissue removal for those new to the technique and is a good measure of surgical proficiency. Being less aggressive anteriorly seems to have an impact on continence. It is often tempting to completely enucleate circumferentially in one continuous plane which is sometimes well beyond the commissure anteriorly. A more moderate dissection in this area can reduce the transient incontinence sometimes seen [3]. The incontinence rates in the current series of 12.8% at 3 months and 2.3% at 12 months are probably representative [1]. An analysis of the factors predisposing to moderate‐to‐severe incontinence in the six patients in this series would have been useful, particularly regarding prostate size, presence of a catheter and age.
The main contribution of HoLEP to the urological armamentarium is its ability to safely treat large prostates endoscopically [4]. Although robot‐assisted techniques have also decreased the morbidity of open prostatectomy [5], the attraction of the obvious ‘natural orifice’ for access and the use of laser technology for the enucleation with HoLEP is probably the least morbid and most cost‐effective way to treat these patients. Tackling a prostate larger than 100 g involves applying the same principles as for smaller prostates, with a few provisos. Firstly, having a consistent strategy for these large prostates is important and can be reassuring when things become difficult. Secondly, it is even more important to maintain the correct plane religiously as it is easier to get lost in these glands. A good sense of direction is important! Thirdly, stay ahead of the bleeding rather than trying to catch up as it can further compound an already difficult situation. Patience is a virtue.
The learning curve of HoLEP has historically been regarded as a major barrier to the uptake of the technique [6]. This has, of course, been exaggerated by proponents of other techniques, but it is important to emphasize that during this learning phase the excellent outcomes are maintained and that conversion to TURP, if necessary (3.4% in this series), can be safely done, as these authors’ have demonstrated. The length of the learning curve has been variously described as being between 20 and 80 cases and is almost entirely due to the way training is done. A modular mentored approach appears to be the best method and could equally be applied to endoscopic enucleation using any of the other energy sources that have been described [7].
HoLEP and all its progeny are here to stay, but which of these enucleation energy sources will gain ascendancy remains to be seen. Sadly, this will likely be more to do with the depth of the corporate pockets and their commitment to the cause rather than proper scientific appraisal [8].
by Peter Gilling
References
- Romero‐Otero J, Garcia‐Gomez B, Garcia‐Gonzalez L et al. Critical analysis of a multicentric experience with holmium laser enucleation of the prostate for benign prostatic hyperplasia: outcomes and complications of 10 years of routine clinical practice. BJU Int 2020; 126: 177-182
- Gilling PJ, Kennett K, Das AK, Thompson D, Fraundorfer MR. Holmium laser enucleation of the prostate (HoLEP) combined with transurethral tissue morcellation: an update on the early clinical experience. J Endourol 1998; 12: 457– 9
- Tunc L, Yalcin S, Kaya E et al. The “Omega Sign”: a novel HoLEP technique that improves continence outcomes after enucleation. World J Urol 2020 https://doi.org/10.1007/s00345-020-03152-9
- Gilling PJ, Kennett KM, Fraundorfer MR. Holmium laser enucleation of the prostate for glands larger than 100 g: an endourologic alternative to open prostatectomy. J Endourol 2000; 14: 529– 31
- Mourmouris P, Keskin SM, Skolarikos A et al. A prospective comparative analysis of robot‐assisted vs open simple prostatectomy for benign prostatic hyperplasia. BJU Int 2019; 123: 313– 7
- Placer J, Gelabert‐Mas A, Vallmanya F et al. Holmium laser enucleation of prostate: outcome and complications of self‐taught learning curve. Urology 2009; 73: 1042– 8
- Kuronen‐Stewart C, Ahmed K, Aydin A et al. Holmium Laser Enucleation of the prostate: simulation based training curriculum and validation. Urology 2015; 86: 639– 46.
- Herrmann TR. Enucleation is enucleation is enucleation is enucleation. World J Urol 2016; 34: 1353– 5