Editorial: Time to re-evaluate and refine re-TUR in bladder cancer?
In this issue of BJUI, Gontero et al. [1] present data from a large multi-centre study that should allow us to re-evaluate and refine the indications for re-transurethral resection (TUR) in bladder cancer.
Herr [2] first described this procedure in 1999 and for the past 16 years the indications have remained largely unchanged and are summarised in the latest European Association of Urology guidelines on non-muscle-invasive bladder cancer (NMIBC) [3]:
- After incomplete initial TUR of bladder tumour (TURBT).
- If there is no muscle in the specimen after initial resection.
- In all T1 tumours.
- In all Grade 3 tumours except primary carcinoma in situ.
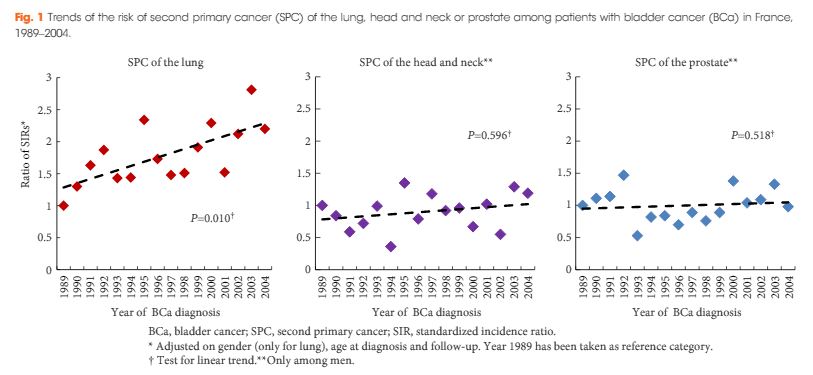
In a multi-centre retrospective study of 2 451 patients with high-grade (HG)/Grade 3 (G3) T1 NMIBC treated with BCG, Gontero et al. [1]examined 935 patients who had re-TUR (38% of the total, itself a low figure). Patients were divided into four groups according to the presence or absence of detrusor muscle in the first TURBT specimen:
- No muscle, no re-TUR
- No muscle, re-TUR
- Muscle, no re-TUR
- Muscle, re-TUR
The authors found that re-TUR only had a positive impact on recurrence, progression, cancer-specific and overall survival, if detrusor muscle was not present in the original specimen. Importantly, in the presence of detrusor muscle in the original specimen, re-TUR did not improve outcomes. The authors conclude that re-TUR may be unnecessary in HG/G3 T1 patients if detrusor muscle is present at the first TURBT.
These findings are important for two reasons: firstly, Herr’s [2] paper was the first to draw attention to the finding that TURBT, a routine urological procedure, was often carried out inadequately. In recent years, the importance of carrying out a high-quality TURBT has been increasingly recognised [4], whilst the presence of detrusor muscle in the TURBT specimen has been shown to be a good measure of the technical quality of a TURBT [5]. This paper [1] further reinforces the importance of obtaining detrusor muscle in the first TURBT. Indeed, as failure to do so results in the patient having to have a second operation and delays their treatment, perhaps we should start to think of a failure to obtain detrusor muscle at the first TURBT in much the same way as positive margin rates are used as a measure of the quality of radical prostatectomy and by inference, the skill of the surgeon.
Secondly, re-TUR arguably serves one overarching purpose: to identify patients with muscle-invasive bladder cancer (MIBC) who have been under-staged by an inadequate first TURBT and who without a re-TUR would be inadequately treated.
Although a secondary role of re-TUR is to identify patients with residual NMIBC, which has some prognostic value, in practice it rarely changes the patient’s management in this setting, which is intravesical therapy usually with BCG. However, in many healthcare systems the timely organisation of a re-TUR within the recommended 6 weeks is challenging and there is usually a further delay of at least 2 weeks until the pathology is reviewed and a patient with NMIBC can finally commence treatment. In this context, it is not surprising that a recent paper in BJUI showed that the interval to re-TUR was a predictor of recurrence and progression and that a re-TUR after 7 weeks was associated with a much worse outcome [6]. It therefore seems logical to reserve re-TUR only for those patients who truly need it, so that limited resources are focused on ensuring that they receive their operation in a timely manner, ideally within 2–4 weeks. If adopted into day-to-day urological practice, the findings by Gontero et al. [1] will allow many patients with HG/G3 T1 and detrusor muscle in the first TURBT specimen to avoid a re-TUR and start intravesical therapy without further delay. Pragmatically, the same should apply to patients with HG/G3 Ta with detrusor muscle in the specimen. On the other hand, HG/G3 T1 patients without detrusor muscle should be fast-tracked for re-TUR as soon as is practicable and certainly no later than 6 weeks.
The article [1] does have some shortcomings. The study design excludes patients with MIBC, so we do not know by comparison how many patients with MIBC were under-staged at the initial TUR based on subsequent re-TUR but as the authors point out, their conclusions would hold true even in this group, as it is very unlikely that one would miss MIBC if there was adequate detrusor muscle in the pathology specimen.
In conclusion, we should consider refining the indications for re-TUR to improve the utilisation of healthcare resources and ensure that for those that need it, a re-TUR is carried promptly whilst for those that do not, essential intravesical treatment is not delayed.