Editorial: Evidence trumps consensus
We read with great interest the article by Khetrapal et al. [1]. Certain advantages of robotic cystectomy have been shown in retrospective studies and confirmed in the RAZOR trial [2]. Robotic cystectomy has been associated with lower blood loss, lower transfusion rates and a shorter length of stay; however, two randomized trials have shown no difference in complication rates, which was the original reason robotic cystectomy was attempted [2,3]. Khetrapal et al. seem to believe that this was because diversions were performed extracorporeally, and intracorporeal diversion would allow urologists to uncover the true benefit of robotic cystectomy. When the RAZOR trial was being designed (in 2009), intracorporeal diversion was early in development. Even today its use in the USA is restricted to a few centres and the Pasadena consensus statement (2015) acknowledges that only 3% of all diversions were performed intracorporeally [4]. While more commonly performed in Europe, intracorporeal diversions still form the minority of all urinary diversions. To date there are no reliable prospective data to convince us that intracorporeal diversion is superior, and the low quality of available evidence has been acknowledged in the Pasadena statement [4]. The iROC trial is a step in the right direction and we await its results with interest [5].
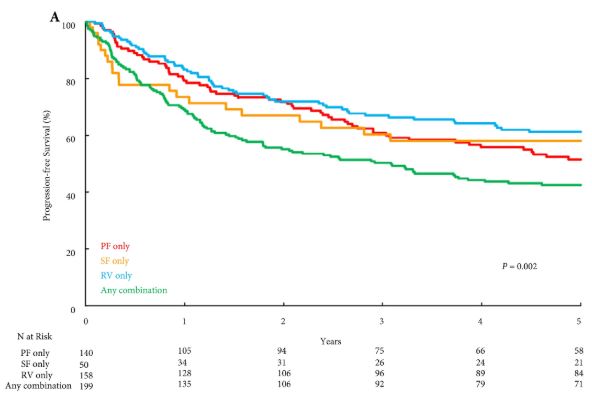
We agree with the authors that cost analysis is essential in evaluating the exact role of robotic cystectomy. It is also worth factoring in the indirect costs of the two procedures, given that most patients undergoing robotic cystectomy will have a shorter hospital stay and fewer blood transfusions, although robotic cystectomies may take longer to perform. We anticipate that as newer robotic systems are introduced the direct surgical costs may be reduced.
There is no universally accepted learning curve for performing a cystectomy based on prospective studies. Ten cystectomies in the preceding year before enrolment in the RAZOR trial was the lowest number of cystectomies permitted for the surgeon to be eligible to participate [2]. All surgeons were fellowship-trained with high-volume bladder cancer practices, and the majority had performed significantly more than 10 cystectomies. The high quality of surgical surrogates for both approaches that we reported, namely, lymph node yield, positive margins and complication rates, are testament to this. We believe that the authors’ statement that novice surgeons may have operated on trial patients is simply inaccurate. It is largely self-serving to fit the results of the RAZOR trial into their own narrative about their beliefs in the advantages of robotic surgery. The iROC trial requires surgeons to have carried out 30 or more intracorporeal diversions in their entire career, with accredited surgeons being required to perform more than 10 cystectomies per year for the last 2 years as primary surgeon, which does not seem remarkably different from the RAZOR trial criteria for surgeon participation [5].While it is clear that large volumes are associated with better outcomes, the magic number is unclear. The Pasadena Consensus Statement cites the National Institute for Health and Care Excellence (NICE) guidelines in the UK, which mandate a minimum of five cystectomies per year per surgeon as adequate surgical volume [4].
Operating time in RAZOR was defined as the time from patient entry to the time the patient exited the operating theatre [2]. In most instances, the time for positioning and anaesthesia (preparation and induction) before making any incision and the time after closure for extubation and leaving the room is generally ~60–80 min. The Pasadena Consensus statement recommends that experienced surgeons should aim to complete robotic cystectomies within 5–6 h, depending on the type of diversion, basing their recommendation on three available studies [4]. Of those papers, Hayn et al. (overall mean operating time 386 min and mean operating time after 50th case 339 min) and Richards et al. (mean operating time 449 min after 40th case of learning curve) defined operating time in their papers as incision to closure time [6,7]. The paper by Collins et al. does not define operating time; however, the mean operating time for cystectomy with intracorporeal diversion for both surgeons in that study was 438 min, and 87.5% of the cases selected in this study had ≤pT2 disease, suggesting a significant selection bias [8]. This institution is a part of the International Robotic Cystectomy Consortium (IRCC) which defines operating time as incision to closure time, leading us to believe that this was the probably the definition they used [8]. A recent study from the IRCC reported a mean operating time (incision to closure) of 364 min in 2134 patients [9]. All these data suggest that operating times in RAZOR were extremely competitive if not actually faster, once again attesting to the proficiency of the participating surgeons. Khetrapal et al. would have reached a different conclusion about the RAZOR trial results had they accurately interpreted the scientific data from the above-mentioned studies.
The RAZOR trial provided level 1 evidence proving the oncological efficacy of robotic cystectomy and confirming advantages such as reduced blood loss and length of stay [2]. We agree that the true place for robotic cystectomy will be determined once a cost–benefit analysis can be performed, and after we obtain high-level prospective data about intracorporeal diversions. To this end, we look forward to the successful completion of the iROC trial and await its publication. Until such time, we suggest more reliance on high-level evidence than on consensus statements and narratives.
by Vivek Venkatramani and Dipen J. Parekh on behalf of RAZOR trial investigators
References
- Khetrapal P, Kelly J, Catto J, Vasdev N. Does the robot have a role in radical cystectomy? BJU Int 2019; 123(3): 380-2.
- ParekhDJ, Reis IM, Castle EP et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an openlabel, randomised, phase 3, non-inferiority trial. Lancet 2018; 391: 2525–36
- Bochner BH, Dalbagni G, Sjoberg DD et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur Urol 2015; 67: 1042–50
- Wilson TG, Guru K, Rosen RC et al. Best practices in robot-assisted radical cystectomy and urinary reconstruction: recommendations of the Pasadena Consensus Panel. Eur Urol 2015; 67: 363–75
- Catto JWF, Khetrapal P, Ambler G et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy (iROC): protocol for a randomised controlled trial with internal feasibility study. BMJ Open 2018; 8: e020500
- Hayn MH, Hussain A, Mansour AM et al. The learning curve of robot- assisted radical cystectomy: results from the international robotic cystectomy consortium. Eur Urol 2010; 58(2): 197–202
- Richards KA, Kader K, Pettus JA et al. Does initial learning curve compromise outcomes for robot-assisted radical cystectomy? A critical evaluation of the first 60 cases while establishing a robotics program. J Endourol 2011; 25(9): 1553–8
- Collins JW, Tyritzis S, Nyberg T et al. Robot-assisted radical cystectomy (RARC) with intracorporeal neobladder – what is the effect of the learning curve on outcomes? BJU Int 2014; 113(1): 100-7
- Hussein AA, May PR, Ahmed YE et al. Development of a patient and institutional-based model for estimation of operative times for robot-assisted radical cystectomy: results from the international robotic cystectomy consortium. BJU Int 2017; 120(5): 695–701



 It's bursting with flavour and with one bite, will melt in your mouth. I’m not just posting this for the laugh – I've created this #rudefood dish in support of @anzuptrials who work tirelessly conducting clinical trials to improve the treatment and outcomes of penile, testicular, bladder, kidney & prostate cancer these cancers are the ones that don’t make the headlines and people don’t want to talk about but we need to change that. Share your #rudefood – food porn with a purpose and use the hashtag #rudefood tagging @anzuptrials And let’s raise awareness for these cancers. #foodpornwithapurpose #cockembouche #anzup
It's bursting with flavour and with one bite, will melt in your mouth. I’m not just posting this for the laugh – I've created this #rudefood dish in support of @anzuptrials who work tirelessly conducting clinical trials to improve the treatment and outcomes of penile, testicular, bladder, kidney & prostate cancer these cancers are the ones that don’t make the headlines and people don’t want to talk about but we need to change that. Share your #rudefood – food porn with a purpose and use the hashtag #rudefood tagging @anzuptrials And let’s raise awareness for these cancers. #foodpornwithapurpose #cockembouche #anzup