Posts
August 2019 – About the cover

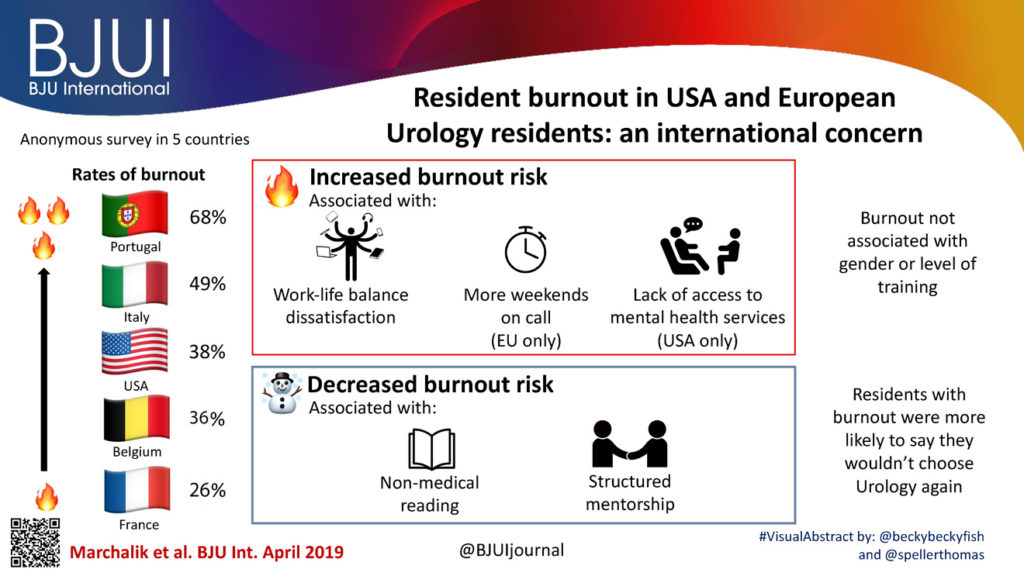
The Article of the Month for August is on work carried out by researchers at the Medstar Georgetown University Hospital, Washington DC, USA along with colleagues from Italy, France and Belgium: Resident burnout in USA and European urology residents: an international concern.
The cover image shows the Lincoln Memorial, which is located in the National Mall in Washington DC. It commemorates the 16th US President, Abraham Lincoln. Washington itself was named after the first US President, George Washington. It lies along the Potomac river and is surrounded by the states of Maryland and Virginia.
© istock.com/Stephen Emlund
Article of the week: Biparametric vs multiparametric prostate MRI for the detection of PCa in treatment‐naïve patients
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community, and a video produced by the authors. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Biparametric vs multiparametric prostate magnetic resonance imaging for the detection of prostate cancer in treatment-naïve patients: a diagnostic test accuracy systematic review and meta-analysis
Mostafa Alabousi*, Jean-Paul Salameh†‡, Kaela Gusenbauer§, Lucy Samoilov¶, Ali Jafri**, Hang Yu§ and Abdullah Alabousi††
*Department of Radiology, McMaster University, Hamilton, †Department of Clinical Epidemiology and Public Health, University of Ottawa, ‡The Ottawa Hospital Research Institute, Clinical Epidemiology Program, Ottawa, §Department of Medicine, McMaster University, Hamilton, ¶Department of Medicine, Western University, London, ON, Canada, **Department of Medicine, New York Institute of Technology School of Osteopathic Medicine, Glen Head, NY, USA, and ††Department of Radiology, St Joseph’s Healthcare, McMaster University, Hamilton, ON, Canada
Abstract
Objective
To perform a diagnostic test accuracy (DTA) systematic review and meta‐analysis comparing multiparametric (diffusion‐weighted imaging [DWI], T2‐weighted imaging [T2WI], and dynamic contrast‐enhanced [DCE] imaging) magnetic resonance imaging (mpMRI) and biparametric (DWI and T2WI) MRI (bpMRI) in detecting prostate cancer in treatment‐naïve patients.
Methods
The Medical Literature Analysis and Retrieval System Online (MEDLINE) and Excerpta Medica dataBASE (EMBASE) were searched to identify relevant studies published after 1 January 2012. Articles underwent title, abstract, and full‐text screening. Inclusion criteria consisted of patients with suspected prostate cancer, bpMRI and/or mpMRI as the index test(s), histopathology as the reference standard, and a DTA outcome measure. Methodological and DTA data were extracted. Risk of bias was assessed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)‐2 tool. DTA metrics were pooled using bivariate random‐effects meta‐analysis. Subgroup analysis was conducted to assess for heterogeneity.
Results
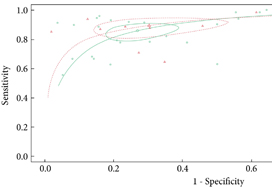
From an initial 3502 studies, 31 studies reporting on 9480 patients (4296 with prostate cancer) met the inclusion criteria for the meta‐analysis; 25 studies reported on mpMRI (7000 patients, 2954 with prostate cancer) and 12 studies reported on bpMRI DTA (2716 patients, 1477 with prostate cancer). Pooled summary statistics demonstrated no significant difference for sensitivity (mpMRI: 86%, 95% confidence interval [CI] 81–90; bpMRI: 90%, 95% CI 83–94) or specificity (mpMRI: 73%, 95% CI 64–81; bpMRI: 70%, 95% CI 42–83). The summary receiver operating characteristic curves were comparable for mpMRI (0.87) and bpMRI (0.90).
Conclusions
No significant difference in DTA was found between mpMRI and bpMRI in diagnosing prostate cancer in treatment‐naïve patients. Study heterogeneity warrants cautious interpretation of the results. With replication of our findings in dedicated validation studies, bpMRI may serve as a faster, cheaper, gadolinium‐free alternative to mpMRI.
Editorial: Dropping the GAD – just a fad?
It is not without irony that, at the very moment that the UK’s National Institute for Health and Care Excellence (NICE) is poised to ratify the recommendation that multiparametric MRI (mpMRI) be introduced into the prostate cancer diagnostic pathway, we are seeking to significantly modify the very intervention on which they are about to provide judgement on [1].
The modification proposed is both compelling and plausible, as it renders the process of imaging the prostate in order to detect and localise clinically significant prostate cancer; simpler, quicker, safer and cheaper. It entails dropping the most complex and time‐consuming component of the three multiparametric sequences, the dynamic (time‐dependent) T1‐weighted gadolinium‐enhanced (GAD) sequence. This was a sequence that was, in the early days of MRI, imbued to have biological significance because it was capable of exploiting the differences in the microvascular architecture and function that we have tended to associate with cancer and non‐cancer in order to discriminate between the two. Or so we thought [2].
The systematic review in this issue of the BJUI by Alabousi et al. [3] explores, via the process of systematic review, whether the omission of the T1‐GAD sequence results in any clinically important reduction in test performance when compared with the full sequence scan comprising traditionally of T2, diffusion and T1‐GAD sequences. It did not.
By any stretch this is a tough analysis to pull‐off, as T1‐GAD sequences are not standardised in terms of acquisition or reporting. Every group seems to manage the dynamic images in a different way. As such they tend to suffer from quality control issues, possibly to a greater extent than the T2 and diffusion sequences. The verification of the signal by biopsy strategy and sampling intensity will have varied across studies, as will the threshold of the definition of clinically significant prostate cancer. These inherent methodological problems are all familiar to readers and issues that are pertinent to any imaging study in the detection of prostate cancer. However, there are two issues that make any current assessment of GAD vs no GAD really problematic. The first is the almost exclusive reliance on single‐centre retrospective data. In the few studies that claim a prospective design no comparative data were available. Studies of this type are typical in the early phase of exploring a clinical question and will, in time, be corrected. The other, largely hidden, hardly discussed and truly problematic issue relates to the manner by which we synthesise an overall risk score from the MRI sequences that we derive. The near ubiquitous use of the Prostate Imaging‐Reporting and Data System (PI‐RADS) scoring system introduces a systematic bias by the manner in which a Boolean form of logic is used to decide on the degree of influence that each sequence has in relation to the overall score. According to the manner by which PI‐RADS is applied, it tends to render the T1‐GAD sequence subordinate (only relevant in a minority of cases), contingent (to T2/diffusion) and disparate (dependent on prostate zone) in the way it is invoked [4]. The result is, that within the PIRADS framework, the T1‐GAD sequence is destined to play a relatively small role in driving the overall summary score of risk. It might, therefore, not be too surprising if its removal made little difference to the overall detection of clinically significant prostate cancer.
So what are the next steps? Clearly this is a very important issue and a simpler, quicker, safer and cheaper MRI would be desirable from multiple perspectives. It would render what is currently a complex intervention that comprises an invasive component into a totally passive image acquisition in which no medically trained health professional need be present. It is almost certainly a pre‐requisite for adoption in resource-poor jurisdictions and for entertaining the role of MRI as a primary population‐based screening test.
It took a large number of randomised trials to get mpMRI accepted into the prostate cancer diagnostic pathway. What is the minimum amount of evidence required to disinvest in one of its key components? In other words how many clinically significant cancers would we tolerate missing in order to offer the less complex test?
A direct (head‐to‐head) non‐inferiority randomised comparative study would, following some of our own recent calculations, require >3000 men to participate, which might just prove a little too challenging. An alternative approach is a study in which men would have lesions declared using a Likert score, thereby making no prior assumptions on the role and utility of any single sequence, by traditional mpMRI (standard) but also by a T2‐diffusion MRI (experimental) with appropriate blinding. Some lesions would be private to either standard or experimental imaging but most, it is likely, would be shared. All would require sampling. The yield, the misses, the test accuracy for each approach, could be calculated with necessary adjustments for the inevitable incorporation and verification biases.
It is interesting to observe that in many parts of the world mpMRI was introduced by clinicians before a large body of evidence was accumulated because they felt it was the right thing to do [5]. It may well be the case that ‘dropping the GAD’ will be subject to the same decision‐making process and precede any definitive judgement based on reliable evidence. Recent activity on PubMed would suggest that this might already have happened [6].
References
- National Institute for Health and Care Excellence (NICE). Non‐invasive MRI scan for Prostate Cancer recommended by NICE. Available at: https://www.nice.org.uk/news/article/non-invasive-mri-scan-for-prostate-cancer-recommended-by-nice. Accessed May 2019.
- , , et al. Evaluation of dynamic contrast‐enhanced MRI biomarkers for stratified cancer medicine: how do permeability and perfusion vary between human tumours? Magn Reson Imaging 2018; 46: 98– 105
- , , et al. Biparametric vs multiparametric prostate magnetic resonance imaging for the detection of prostate cancer in treatment‐naïve patients: a diagnostic test accuracy systematic review and meta‐analysis. BJU Int 2019; 124: 209– 20
- , , et al. Prostate Imaging reporting and Data System version 2.1: 2019 update of Prostate Imaging Reporting and Data System version 2. Eur Urol 2019 [Epub ahead of print]. https://doi.org/10.1016/j.eururo.2019.02.033
- , , et al. Is it time to consider a role for MRI before prostate biopsy? Nat Rev Clin Oncol 2009; 6: 197– 20
- , , et al. Using biparametric MRI radiomics signature to differentiate between benign and malignant prostate lesions. Eur J Radiol 2019; 114: 38– 44
Video: Biparametric vs multiparametric prostate MRI for the detection of PCa in treatment‐naïve patients: a diagnostic test accuracy systematic review and meta‐analysis
Abstract
Objective
To perform a diagnostic test accuracy (DTA) systematic review and meta‐analysis comparing multiparametric (diffusion‐weighted imaging [DWI], T2‐weighted imaging [T2WI], and dynamic contrast‐enhanced [DCE] imaging) magnetic resonance imaging (mpMRI) and biparametric (DWI and T2WI) MRI (bpMRI) in detecting prostate cancer in treatment‐naïve patients.
Methods
The Medical Literature Analysis and Retrieval System Online (MEDLINE) and Excerpta Medica dataBASE (EMBASE) were searched to identify relevant studies published after 1 January 2012. Articles underwent title, abstract, and full‐text screening. Inclusion criteria consisted of patients with suspected prostate cancer, bpMRI and/or mpMRI as the index test(s), histopathology as the reference standard, and a DTA outcome measure. Methodological and DTA data were extracted. Risk of bias was assessed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)‐2 tool. DTA metrics were pooled using bivariate random‐effects meta‐analysis. Subgroup analysis was conducted to assess for heterogeneity.
Results
From an initial 3502 studies, 31 studies reporting on 9480 patients (4296 with prostate cancer) met the inclusion criteria for the meta‐analysis; 25 studies reported on mpMRI (7000 patients, 2954 with prostate cancer) and 12 studies reported on bpMRI DTA (2716 patients, 1477 with prostate cancer). Pooled summary statistics demonstrated no significant difference for sensitivity (mpMRI: 86%, 95% confidence interval [CI] 81–90; bpMRI: 90%, 95% CI 83–94) or specificity (mpMRI: 73%, 95% CI 64–81; bpMRI: 70%, 95% CI 42–83). The summary receiver operating characteristic curves were comparable for mpMRI (0.87) and bpMRI (0.90).
Conclusions
No significant difference in DTA was found between mpMRI and bpMRI in diagnosing prostate cancer in treatment‐naïve patients. Study heterogeneity warrants cautious interpretation of the results. With replication of our findings in dedicated validation studies, bpMRI may serve as a faster, cheaper, gadolinium‐free alternative to mpMRI.
Article of the week: A transcriptomic signature of tertiary Gleason 5 predicts worse clinicopathological outcome
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community, and a video produced by the authors. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
A transcriptomic signature of tertiary Gleason 5 predicts worse clinicopathological outcome
Abstract
Objective
To investigate the genomic features of tertiary pattern 5 (TP5) on radical prostatectomy specimens in an effort to explain the poor clinical outcomes associated with this disease subtype.
Patients and methods
Data from 159 men with Gleason Grade Group (GGG) 3 or 4 were considered. All patients had Decipher diagnostic testing with transcript profiles and single‐channel array normalisation (SCAN)‐normalised expression of coding genes.
The relationship between Decipher and TP5 was investigated by linear and binary logistic regressions. A differential transcriptomic analysis between patients with and without TP5 was performed. The prognostic role of these genes on progression‐free survival (PFS) and overall survival (OS) was evaluated using The Cancer Genome Atlas.
Results
In all, 52/159 (33%) patients had GGG 3–4 with TP5 disease. TP5 was associated with a higher Decipher score (β 0.07, 95% confidence interval [CI] 0.02–0.13; P = 0.04) and higher likelihood of falling within the intermediate‐ or high‐risk categories (odds ratio 3.34, 95% CI 1.34–8.35; P = 0.01). Analysis of microarray data revealed an 18‐gene signature that was differentially expressed in patients with TP5; 13 genes were over‐ and five under‐expressed in the TP5 cohort.
The overexpression of cyclin dependent kinase inhibitor 2B (CDKN2B), polo‐like kinase 1 (PLK1), or cell division cycle 20 (CDC20) was associated with worse PFS. The group harbouring overexpression of at least one gene had a 5‐year PFS rate of 50% vs 74% in the group without overexpression (P < 0.001).
Conclusions
Our studies have elucidated unique genomic features of TP5, whilst confirming previous clinical findings that patients harbouring TP5 tend to have worse prognosis. This is the first RNA‐based study to investigate the molecular diversity of TP5 and the first correlating CDKN2B to poorer prognosis in patients with prostate cancer.
Editorial: Transcriptomic signatures for tertiary pattern 5 in prostate cancer
Significant evidence is now emerging and publications have shown that the molecular characterization of tumours represents an important approach to stratifying patients with prostate cancer into appropriate treatments and their responses, helping to inform the next steps in the care pathway of these patients [1]. Understanding the functional role of the genes included in these signatures also gives an insight into the biology of the disease and how it develops and progresses.
BJUI has published papers in the past describing gene signatures that are associated with pathological Gleason score as pathological markers of aggressiveness and their potential role in predicting outcome [2].
The paper by Martini et al. [3] in the current issue of BJUI adds to this important literature. In a cohort of 159 patients, of whom 52 had tertiary pattern 5 (TP5) prostate cancer, Martini et al. showed that TP5 was associated with higher risk categories in the Decipher genomic score. Their study supports the literature showing that patients with TP5 have an elevated likelihood of developing disease after radical prostatectomy and have a poor prognosis [4]. It should be noted, however, that this is a small cohort with few patients defined as having TP5 disease. As prostate cancer is highly heterogeneous and gene expression can also be affected by the patient’s background, additional validation of this finding will be required in independent cohorts.
Additional analysis of the 698 gene by Martini et al. [3] identified a unique RNA signature of 18 genes for the TP5 cohort. A limitation of using the 698 gene previously demonstrated to be related to prostate cancer is that those with TP5 disease may be a unique subset of patients that may be associated with unfamiliar pathological pathways and new genes. An examination of novel genes whose expression pattern is unique to patients with TP5 disease may reveal additional genes.
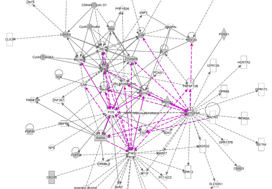
Martini et al. then used the independent TCGA provisional database to link the expression of these 18 genes with clinical features. Patients harbouring any of the three genes CDKN2B, PLK1 and CDC20 mRNA were found to have worse progression‐free survival. The authors go on to undertake analysis of the 18 genes using the ingenuity network analysis tool identifying pathways involved in the cell cycle, DNA replication and repair, cellular assembly, cell death and survival and gene expression, which would fit with the biology of progressive disease. They also revealed a role in the dedifferentiation process and progression of cells towards anaplasia. They specifically identified CDKN2B, which has previously been associated with tumour suppression but might actually be linked to tumour progression, and identified it for further investigation.
Finally the researchers found that 13 genes out of the 18‐gene TP5 signature are upregulated in tumour lesions with TP5. Following these results they performed a sensitivity analysis in which the expression values of the genes in the cohort with GGG3‐4 and TP5 were compared with those of patients with GGG5 and discovered that these genes were similarly overexpressed in both groups. This finding strengthens the hypothesis that these genes are implicated in the dedifferentiation process of tumour cells, but it also raises the question of whether the pattern of expression of these genes is unique to patients with TP5 only, or whether it is associated with every tumour (primary, secondary or tertiary) that is classified as GGG5. Further research is needed to understand what the clinical significance of this genetic signature is and what pathological process it describes in practice.
This investigation into the functional role of the genes has given an insight into the biology of prostate cancer progression and TP5 disease which might inform a future area of research for novel biomarker panels and therapeutic interventions.
by R. William Watson and Omri Taltsh
References
- , , , , , , Genomic markers in prostate cancer decision making. Euro Urol 2018; 73: 572– 82
- , , , , , , , ,, , , , Evaluation of a 24‐Gene signature for prognosis of metastatic events and prostate cancer‐specific mortality. BJU Int 2017; 119: 961– 7
- , , , , , , ,, , A transcriptomic signature of tertiary Gleason 5 predicts worse clinicopathological outcome. BJU Int 2019; 124: 155– 62
- , , , , , Significance of tertiary Gleason pattern 5 in Gleason score 7 radical prostatectomy specimens. J Urol 2008; 179:516– 22
Video: A transcriptomic signature of tertiary Gleason 5 predicts worse clinicopathological outcome
A transcriptomic signature of tertiary Gleason 5 predicts worse clinicopathological outcome
by Alberto Martini
Abstract
Objective
To investigate the genomic features of tertiary pattern 5 (TP5) on radical prostatectomy specimens in an effort to explain the poor clinical outcomes associated with this disease subtype.
Patients and methods
Data from 159 men with Gleason Grade Group (GGG) 3 or 4 were considered. All patients had Decipher diagnostic testing with transcript profiles and single‐channel array normalisation (SCAN)‐normalised expression of coding genes.
The relationship between Decipher and TP5 was investigated by linear and binary logistic regressions. A differential transcriptomic analysis between patients with and without TP5 was performed. The prognostic role of these genes on progression‐free survival (PFS) and overall survival (OS) was evaluated using The Cancer Genome Atlas.
Results
In all, 52/159 (33%) patients had GGG 3–4 with TP5 disease. TP5 was associated with a higher Decipher score (β 0.07, 95% confidence interval [CI] 0.02–0.13; P = 0.04) and higher likelihood of falling within the intermediate‐ or high‐risk categories (odds ratio 3.34, 95% CI 1.34–8.35; P = 0.01). Analysis of microarray data revealed an 18‐gene signature that was differentially expressed in patients with TP5; 13 genes were over‐ and five under‐expressed in the TP5 cohort.
The overexpression of cyclin dependent kinase inhibitor 2B (CDKN2B), polo‐like kinase 1 (PLK1), or cell division cycle 20 (CDC20) was associated with worse PFS. The group harbouring overexpression of at least one gene had a 5‐year PFS rate of 50% vs 74% in the group without overexpression (P < 0.001).
Conclusions
Our studies have elucidated unique genomic features of TP5, whilst confirming previous clinical findings that patients harbouring TP5 tend to have worse prognosis. This is the first RNA‐based study to investigate the molecular diversity of TP5 and the first correlating CDKN2B to poorer prognosis in patients with prostate cancer.