Article of the week: Characterising ‘bounce‐back’ readmissions after radical cystectomy
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urology community and a visual abstract prepared by a creative urologist; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Characterising ‘bounce‐back’ readmissions after radical cystectomy
Peter S. Kirk*, Ted A. Skolarus*†, Bruce L. Jacobs‡, Yongmei Qin*, Benjamin Li*, Michael Sessine*, Xiang Liu§, Kevin Zhu*, Scott M. Gilbert¶, Brent K. Hollenbeck*, Ken Urish**, Jonathan Helm††, Mariel S. Lavieri§ and Tudor Borza‡‡
*Dow Division of Health Services Research, Department of Urology, University of Michigan Health System, Ann Arbor, MI, USA, †VA Health Services Research and Development, Center for Clinical Management Research, VA Ann Arbor Healthcare System, Ann Arbor, MI, USA, ‡Department of Urology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA, §Department of Industrial and Operations Engineering, University of Michigan, Ann Arbor, MI, USA, ¶Department of Genitourinary Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA, **Department of Orthopedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA, ††Department of Operations and Decision Technologies, Kelley School of Business, Indiana University, Bloomington, IN, USA, and ‡‡Department of Urology, University of Wisconsin, Madison, WI, USA
Abstract
Objective
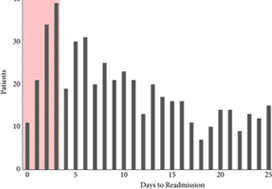
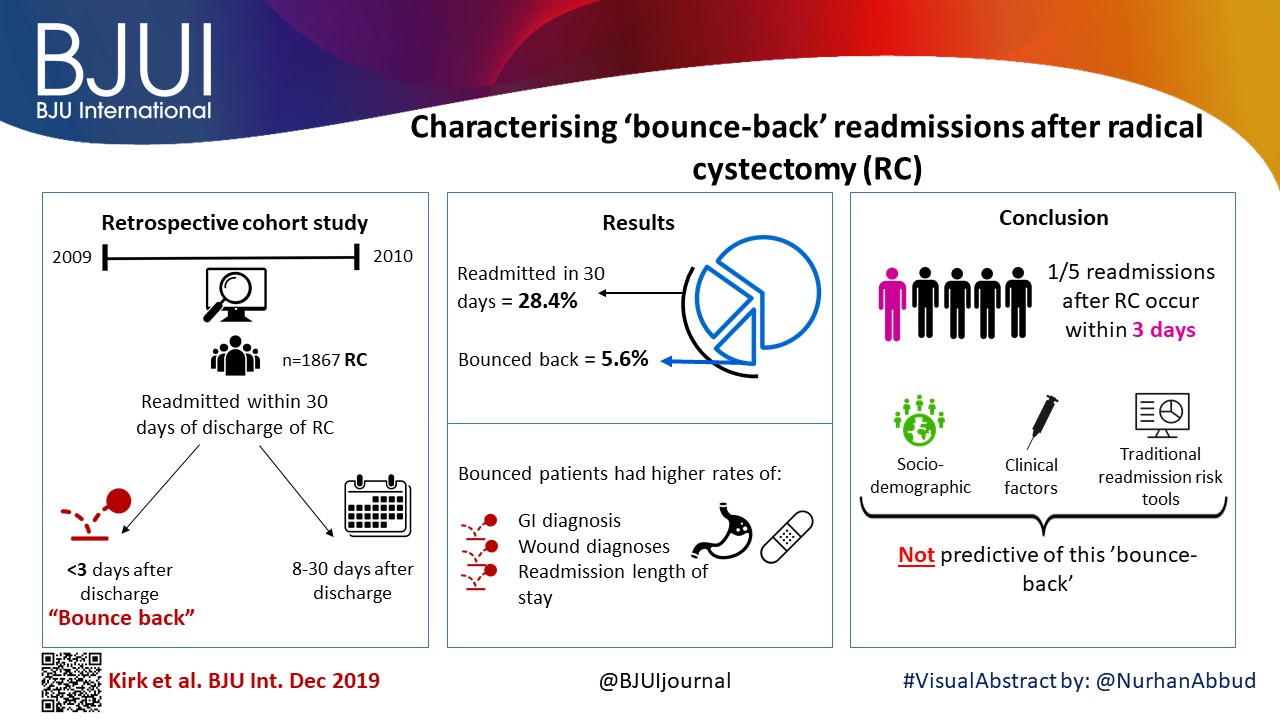
To examine predictors of early readmissions after radical cystectomy (RC). Factors associated with preventable readmissions may be most evident in readmissions that occur within 3 days of discharge, commonly termed ‘bounce‐back’ readmissions, and identifying such factors may inform efforts to reduce surgical readmissions.
Patients and Methods
We utilised the Healthcare Cost and Utilization Project’s State Inpatient Databases to examine 1867 patients undergoing RC in 2009 and 2010, and identified all patients readmitted within 30 days of discharge. We assessed differences between patients experiencing bounce‐back readmission compared to those readmitted 8–30 days after discharge using logistic regression models and also calculated abbreviated LACE scores to assess the utility of common readmissions risk stratification algorithms.
Results
The 30‐day and bounce‐back readmission rates were 28.4% and 5.6%, respectively. Although no patient or index hospitalisation characteristics were significantly associated with bounce‐back readmissions in adjusted analyses, bounce‐back patients did have higher rates of gastrointestinal (14.3% vs 6.7%, P = 0.02) and wound (9.5% vs 3.0%, P < 0.01) diagnoses, as well as increased index and readmission length of stay (5 vs 4 days, P = 0.01). Overall, the median abbreviated LACE score was 7, which fell into the moderate readmission risk category, and no difference was observed between readmitted and non‐readmitted patients.
Conclusion
One in five readmissions after RC occurs within 3 days of initial discharge, probably due to factors present at discharge. However, sociodemographic and clinical factors, as well as traditional readmission risk tools were not predictive of this bounce‐back. Effective strategies to reduce bounce‐back readmission must identify actionable clinical factors prior to discharge.