Video: Accuracy of ultrasonography for renal stone detection and size determination: is it good enough for management decisions?
Accuracy of ultrasonography for renal stone detection and size determination: is it good enough for management decisions?
Abstract
Objectives
To determine the sensitivity and specificity of ultrasonography (US) for detecting renal calculi and to assess the accuracy of US for determining the size of calculi and how this can affect counselling decisions.
Materials and Methods
We retrospectively identified all patients at our institution with a diagnosis of nephrolithiasis who underwent US followed by non-contrast computed tomography (CT) within 60 days. Data on patient characteristics, stone size (maximum axial diameter) and stone location were collected. The sensitivity, specificity and size accuracy of US was determined using CT as the standard.
Results
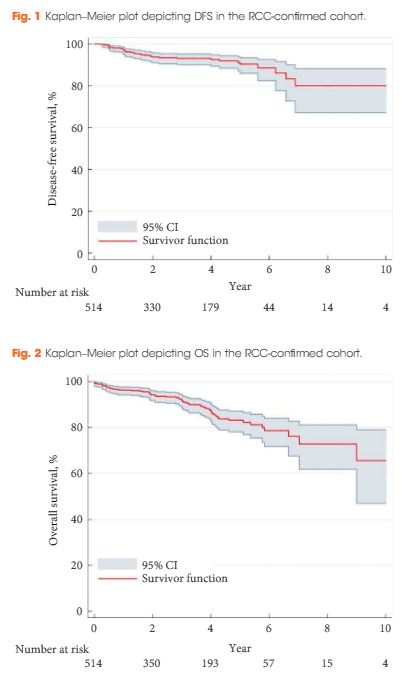
A total of 552 US and CT examinations met the inclusion criteria. Overall, the sensitivity and specificity of US was 54 and 91%, respectively. There was a significant association between sensitivity of US and stone size (P < 0.001), but not with stone location (P = 0.58). US significantly overestimated the size of stones in the 0–10 mm range (P < 0.001). Assuming patients with stones 0–4 mm in size will be selected for observation and those with stones ≥5 mm could be counselled on the alternative of intervention, we found that in 14% (54/384) of cases where CT would suggest observation, US would lead to a recommendation for intervention. By contrast, when CT results would suggest intervention as management, US would suggest observation in 39% (65/168) of cases. An average of 22% (119/552) of patients could be inappropriately counselled. Stones classified as 5–10 mm according to US had the highest probability (43% [41/96]) of having their management recommendation changed when CT was performed. The use of plain abdominal film of kidney, ureter and bladder and US increases sensitivity (78%), but 37% (13/35) of patients may still be counselled inappropriately to undergo observation.
Conclusions
Using US to guide clinical decision-making for residual or asymptomatic calculi is limited by low sensitivity and inability to size the stone accurately. As a result, one in five patients may be inappropriately counselled when using US alone.