Article of the Week: Ureteric stent dwelling time: a risk factor for post-ureteroscopy sepsis
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Ureteric stent dwelling time: a risk factor for post-ureteroscopy sepsis
Abstract
Objectives
To evaluate the association between stent dwelling time and sepsis after ureteroscopy, and identify risk factors for sepsis in this setting.
Patients and Methods
The prospectively collected database of a single institution was queried for all patients who underwent ureteroscopy for stone extraction between 2010 and 2016. Demographic, clinical, preoperative and operative data were collected. The primary study endpoint was sepsis within 48 h of ureteroscopy. Logistic regressions were performed to identify predictors of post-ureteroscopy sepsis in the ureteroscopy cohort and specifically in patients with prior stent insertion.
Results
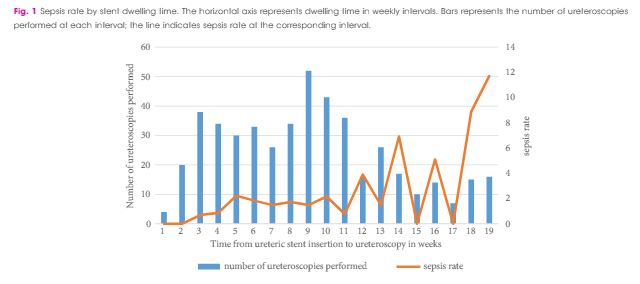
Between October 2010 and April 2016, 1 256 patients underwent ureteroscopy for stone extraction. Risk factors for sepsis included prior stent placement, female gender and Charlson comorbidity index. A total of 601 patients had a ureteric stent inserted before the operation and were included in the study cohort, in which the median age was 56 years, 90 patients were women (30%), and 97 patients were treated for positive preoperative urine cultures (16.1%). Postoperative sepsis, <48 h after surgery, occurred in eight (1.2%) non-stented patients and in 28 patients (4.7%) with prior stent insertion. Sepsis rates after stent dwelling times of 1, 2, 3 and >3 months were 1, 4.9, 5.5 and 9.2%, respectively. On multivariate analysis, stent dwelling time, stent insertion because of sepsis, and female gender were significantly associated with post-ureteroscopy sepsis in patients with prior stent placement.
Conclusions
Patients who undergo ureteroscopy after ureteric stent insertion have a higher risk of postoperative sepsis. Prolonged stent dwelling time, sepsis as an indication for stent insertion, and female gender are independent risk factors. Stent placement should be considered cautiously, and if inserted, ureteroscopy should be performed within 1 month.