Editorial: Prostate cancer biomarkers: new scenarios in the multi-parametric magnetic resonance imaging era
The management of prostate cancer poses difficult challenges, which is largely because we lack the necessary tools to predict its presence, and discern between indolent disease with a small chance of clinical manifestation and aggressive tumours that are more likely to be lethal.
Despite the fact that novel blood and urine tests are available, which may predict aggressive disease better than PSA; they are not routinely used due to a lack of clinical validity studies.
Tosoian et al. [1] in the present study explored the utility of prostate health index (PHI) density for detection of clinically significant prostate cancer in a contemporary cohort of men presenting for diagnostic evaluation of prostate cancer. Very interestingly the authors hypothesised that, similar to PSA density, PHI density could further improve upon the discriminative ability of PHI to detect prostate cancer. The PHI density calculation was performed using prostate volume, as determined by TRUS. Logistic regression was used to assess the ability of serum markers to predict clinically significant prostate cancer, defined as any Gleason score ≥7 cancer or Gleason score 6 cancer in >2 cores or >50% of any positive core.
They showed, albeit in a small sample size, that PHI density could further improve upon the discriminative ability of PHI and appears to be superior to PSA and other PSA derivatives for the identification of clinically significant disease [1].
However, it is noteworthy that in all studies on urine or serum biomarkers such as this, the ‘gold standard’ for cancer detection is pathological examination of multiple non-targeted systematic TRUS-guided prostate biopsies, not radical prostatectomy specimens. Intrinsically, this approach implies that no cancer predicted by the biomarker may still mean cancer missed by the biopsy.
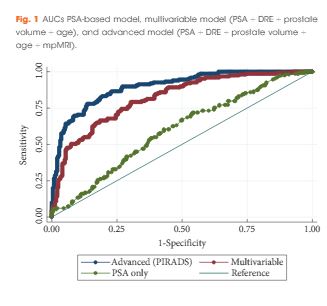
Recently, the European Association of Urology Guidelines have introduced multi-parametric MRI (mpMRI) as a strongly recommended examination before repeating biopsy when clinical suspicion of prostate cancer persists [2]. Indeed, mpMRI is very accurate in detecting clinically significant prostate cancer, with a positive predictive value of 82% and a negative predictive value of >95% for excluding high-risk prostate cancer [3, 4].
Introducing mpMRI before prostate biopsy has the potential to improve prostate cancer sampling ink that is the most practical way to make mpMRI before biopsy economically viable for universal NHS adoption.
The aim should be the development of a clinical decision support system based on mpMRI and circulating biomarkers, as in this case PHI density evaluation, to stratify patients according to their risk of prostate cancer progression, using pathological assessment after prostatectomy as the reference standard.