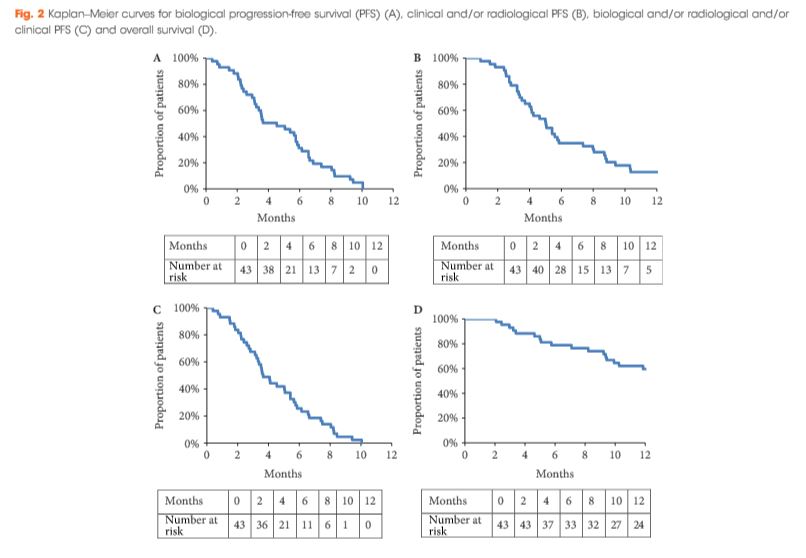
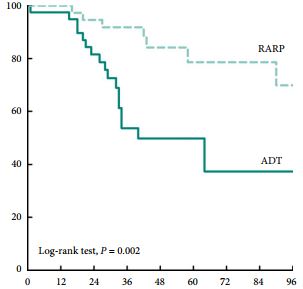
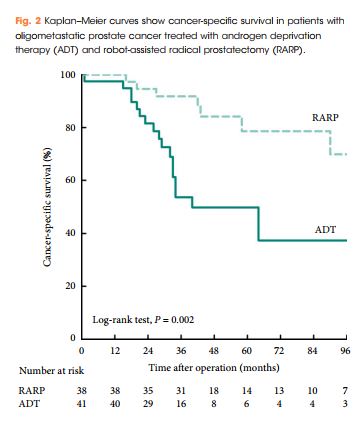
Editorial: Is overall survival not influenced by PN vs RN?
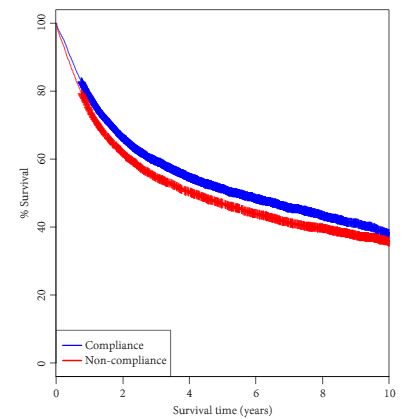
In this issue of the BJUI, Abdollah et al. [1] have for the first time tested the external validity of the only randomized clinical trial, 30904, run by the European Organization for the Research and Treatment of Cancer Genito-Urinary Group (EORTC GU) in the early 1990s, comparing cancer-specific survival and overall survival in patients with solitary renal masses of ≤5 cm and stage T1 and T2 in the TNM classification (in use at that time). The trial showed, as expected, that renal function was worse after radical nephrectomy (RN) and that the complication rate was higher after partial nephrectomy (PN) [2]. However, unexpectedly, overall survival after PN was not better than after RN [3], as was suggested or claimed in many non-randomized studies and also in a meta-analysis that included the EORTC 30904 trial as the only randomized clinical trial [4].
Despite a couple of limitations in the randomized trial, and it’s premature closure because of slow accrual, we performed a second analysis looking at the estimated GRF in the vast majority of the included patients and, most importantly, showed that kidney function did not progressively deteriorate after RN when the contralateral kidney was normal, and that only exceptionally did patients developed chronic kidney disease (CKD) necessitating dialysis [5].
Whilst it was anticipated that decreased kidney function should induce cardiovascular disease and increase cardiovascular death, this was separately investigated by Capitanio et al. [6] in a multicentre study where this suggestion was confirmed. However, looking at their Kaplan–Meier curves, it is clear that, although the negative impact on cardiovascular disease should become more and more obvious and accumulate over time, the split of the curves in favor of PN occurred very early after surgery. This indicates that the patients included in these non-randomized studies were different from the start, meaning that those selected for PN were ‘better’ patients who obviously had less cardiovascular disease and therefore had better cardiovascular outcomes. Another study confirmed that both PN and RN impact on cardiovascular disease [7], whilst another meta-analysis showed no difference for cardiovascular outcomes [8]. Obviously patients with preoperative CKD will benefit from nephron-sparing surgery [9], as well as those who have concomitant conditions, e.g. hypertension, diabetes, and a worse Charlson’s Comorbidity Index [10].
The authors, who tested the external validity of the EORTC 30904 trial in contemporary North American patients, need to be congratulated for the effort undertaken to show that the EORTC 30904 cohort was not significantly different from the National Cancer Database cohort in a manner that could influence the reported trial outcomes.