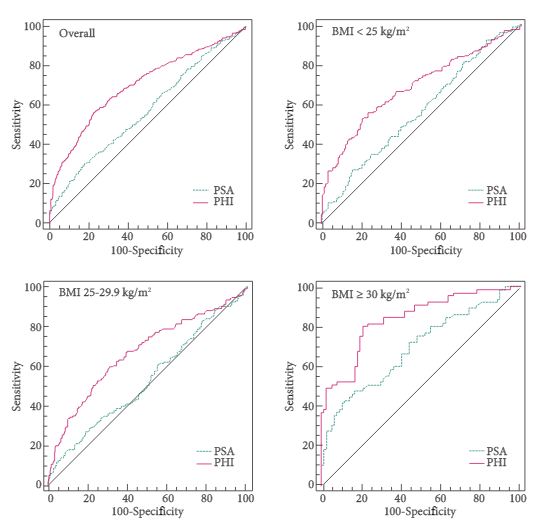
Enzalutamide is a second generation non-steroidal antiandrogen (AA), which significantly improved overall survival (OS) and progression-free survival (PFS) after docetaxel (AFFIRM study), and OS and radiographic PFS before chemotherapy (PREVAIL study) in patients with metastatic castration-resistant prostate cancer (mCRPC) [1].
Being a potent androgen receptor (AR) antagonist, an enzalutamide withdrawal syndrome (EWS) appeared unlikely [2,3]. In contrast with this position, and considering the well-known AR structural alterations in CRPC, very recent preclinical and clinical data support the possibility of the existence of an EWS after the discontinuation of this drug, in a castration-resistant setting.
In patients with mCRPC progressing on androgen-deprivation therapy (ADT), the AAWS, due to the interruption of first generation AAs (flutamide, bicalutamide, nilutamide, cyproterone acetate and megestrol acetate), often pursues as a further hormonal manipulation, although no level one studies supported its efficacy. AAWS leads to a PSA reduction in 15–30% of patients, with concomitant symptomatic relief and radiographic responses in some cases, without impacting on survival [1]. The molecular mechanisms of the AAWS are still unclear. One of the possible mechanisms responsible for the AAWS is the mutation of the AR. In vitro models showed that bicalutamide may switch from antagonist to agonist in LNCaP-cxD cell lines, due to an additional AR mutation in codon 741 during bicalutamide treatment [4]. Similarly, preclinical in vitro and in vivo studies demonstrated that initially enzalutamide exerts its AR antagonist activity, but during the treatment enzalutamide potently induces an AR mutation, leading to the mutant ARF876L, which confers agonism to enzalutamide, and resistance to enzalutamide therapy [1]. In the clinical setting, the first evidence of possible EWS has begun to appear. Phillips [5] observed EWS in one patient, 40 days after enzalutamide discontinuation. Rodriguez-Vida et al. [1] showed EWS in three of 30 (10%) patients with mCRPC after the drug cessation, although none of the 17 factors examined were statistically significant predictors of PSA decline after enzalutamide interruption. Considering the clinical characteristics of the three patients showing EWS, interestingly all of them were aged <70 years, had Gleason score ≥7, had bone and/or lymph node metastases without visceral sites of disease, and had had previous treatments with bicalutamide and docetaxel. In all three patients, EWS seems to have no correlation with: prostate cancer staging at diagnosis (M0 vs M1), PSA value before enzalutamide, PSA decline on enzalutamide treatment, LHRH analogues and enzalutamide therapy duration. Similarly to bicalutamide WS, in all three patients no symptomatic improvement was recorded during EWS.
Focusing on patient 1, interestingly he displayed initially a PSA response during enzalutamide and later a further PSA decline plus radiological response after enzalutamide interruption (i.e. EWS), showing a sensitivity either to initial enzalutamide antagonism and to subsequent agonism, and exhibiting an EWS not preceded by a bicalutamide WS. This supports previous data concerning different AR mutations for the two different AAs, without subsequent clinical correlations between the two different AAWSs. A LHRH analogue duration treatment (5 months) shorter than a subsequent enzalutamide duration therapy (21.4 months) suggests different tumoral cells sensitivity to different ADTs, in different stages (hormone naïve and castration-resistant prostate cancer), likely related to several AR structural alterations collected along the disease. Intriguingly, patient 3 discontinued enzalutamide after only 1.2 months, maybe due to primary resistance [6]; nevertheless, he showed a PSA
response after enzalutamide interruption, suggesting that EWS, characterised by the switch from enzalutamide antagonism to agonism, could occur even in prostate cancer patients primary resistant to this drug. Limitations of the two first clinical reports related to EWS include a restricted sample size, a reduced number of EWS events and a short duration of follow-up after enzalutamide discontinuation. Furthermore, preclinical studies on EWS and published data concerning AAWS described more frequently early stage mCRPC, while the two papers considered heavily pretreated patients with mCRPC, in whom EWS incidence could be reduced due to several previous treatments, which probably produced various AR alterations.
In conclusion, the preclinical models have demonstrated one possible plausible mechanism responsible for EWS and enzalutamide resistance. First clinical reports suggest the possibility of EWS in a minority of patients after enzalutamide discontinuation in mCRPC. Further studies are needed to confirm and detail the EWS, before translating these data into clinical practice.
Alessandra Mosca
Medical Oncology, ‘Maggiore della Carità’ University Hospital, Novara, Italy
References
1 Rodriguez-Vida A, Bianchini D, Van Hemelrijck M et al. Is there an antiandrogen withdrawal syndrome with enzalutamide? BJU Int 2015; 115: 373–80
2 von Klot CA, Kramer MW, Böker A et al. Is there an anti-androgen withdrawal syndrome for Enzalutamide? World J Urol 2014; 32: 1171–6
3 von Klot CA, Kuczyk MA, Merseburger AS. No androgen withdrawal syndrome for enzalutamide: a report of disease dynamics in the postchemotherapy setting. Eur Urol 2014; 65: 258–9
4 Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res 2003; 63: 149–53
5 Phillips R. An enzalutamide antiandrogen withdrawal syndrome. Nat Rev Urol 2014; 11: 366
6 Efstathiou E, Titus M, Wen S et al. Molecular characterization of Enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol 2015; 67: 53–60