Every Month the Editor-in-Chief selects the Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Month heading on the homepage will consist of additional material or media. This week we feature a video from Dr. Anna Gavin discussing his paper.
If you only have time to read one article this week, it should be this one.
Patient reported “ever had” and “current” long term physical symptoms following prostate cancer treatments.
Anna T. Gavin, Frances J. Drummond*, Conan Donnelly, Eamonn O’Leary*, Linda Sharp† and Heather R. Kinnear
Northern Ireland Cancer Registry, Centre for Public Health, Queen’s University Belfast, Mulhouse Building, Belfast Northern Ireland, UK, *National Cancer Registry Ireland, Building 6800, Airport Business Park Cork, Ireland, and †Institute of Health and Society, Newcastle University, Richardson Road, Newcastle upon Tyne, NE2 4AX, England, UK
OBJECTIVE
To investigate the prevalence of physical symptoms that were ‘ever’ and ‘currently’ experienced by survivors of prostate cancer at a population level, to assess burden and thus inform policy to support survivors.
PATIENTS AND METHODS
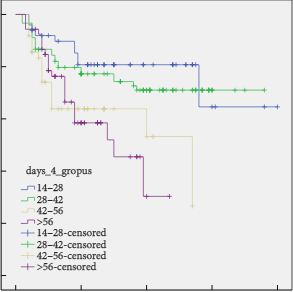
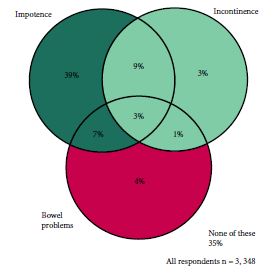
The study included 3 348 men surviving prostate cancer for 2–18 years after diagnosis. A cross-sectional, postal survey of 6 559 survivors diagnosed 2–18 years ago with primary, invasive prostate cancer (ICD10-C61) identified via national, population-based cancer registries in Northern Ireland and Republic of Ireland. Questions included symptoms at diagnosis, primary treatments and physical symptoms (erectile dysfunction [ED]/urinary incontinence [UI]/bowel problems/breast changes/loss of libido/hot flashes/fatigue) experienced ‘ever’ and at questionnaire completion (‘current’). Symptom proportions were weighted by age, country and time since diagnosis. Bonferroni corrections were applied for multiple comparisons.
RESULTS
Adjusted response rate 54%; 75% reported at least one ‘current’ physical symptom (‘ever’ 90%), with 29% reporting at least three. Prevalence varied by treatment. Overall, 57% reported current ED and this was highest after radical prostatectomy (RP, 76%) followed by external beam radiotherapy with concurrent hormone therapy (HT, 64%). UI (overall ‘current’ 16%) was highest after RP (‘current’ 28%; ‘ever’ 70%). While 42% of brachytherapy patients reported no ‘current’ symptoms, 43% reported ‘current’ ED and 8% ‘current’ UI. ‘Current’ hot flashes (41%), breast changes (18%) and fatigue (28%) were reported more often by patients on HT.
CONCLUSION
Symptoms after prostate cancer treatment are common, often multiple, persist long-term and vary by treatment method. They represent a significant health burden. An estimated 1.6% of men aged >45 years are survivors of prostate cancer and currently experiencing an adverse physical symptom. Recognition and treatment of physical symptoms should be prioritised in patient follow-up. This information should facilitate men and clinicians when deciding about treatment as differences in survival between radical treatments is minimal.
 The authors of January’s BJUI Article of The Month come from Hamburg and Mannheim in Germany. The cover shows Hamburg’s Speicherstadt, the largest warehouse district in the world and a UNESCO World Heritage Site.
The authors of January’s BJUI Article of The Month come from Hamburg and Mannheim in Germany. The cover shows Hamburg’s Speicherstadt, the largest warehouse district in the world and a UNESCO World Heritage Site.