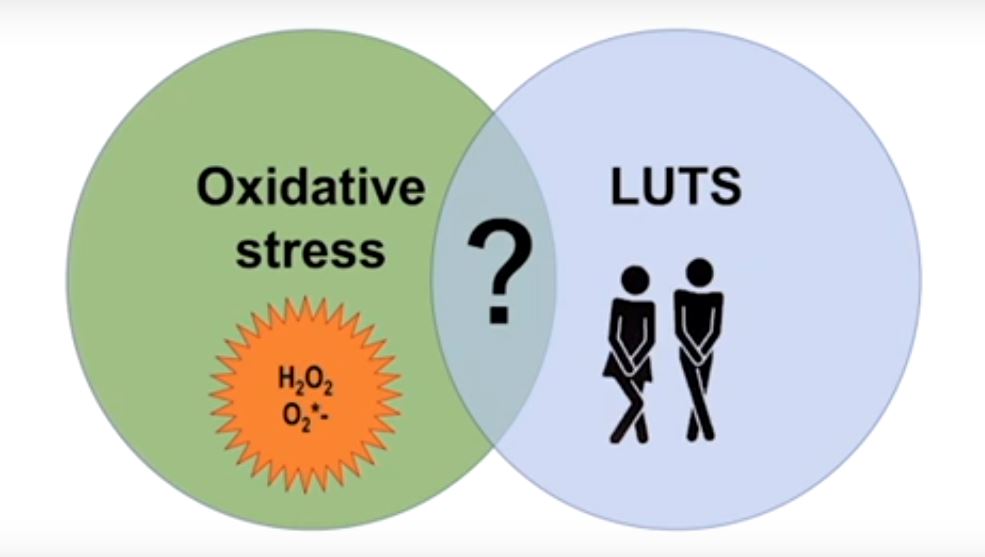
Oxidative stress has been defined as ‘an imbalance between oxidants and anti-oxidants in favour of the oxidants, leading to a disruption of redox signalling and control and/or molecular damage’ [1]. Reactive oxygen and nitrogen species (ROS/RNS) produced under oxidative stress are known to damage all cellular biomolecules (lipids, sugars, proteins and polynucleotides). ROS/RNS is often used as a generic term but it has been emphasized that all ROS/RNS molecules are not the same [2] and the term encompasses a diverse range of species, including, for example, superoxide, hydrogen peroxide, nitric oxide and peroxynitrite. The biological impacts of ROS/RNS depend critically on the particular molecule(s) involved, and on the microenvironment and physiological or pathological context in which it is being generated [2]. It should be emphasized that ROS are not only harmful agents that cause oxidative damage in pathologies but they also have important roles as regulatory agents in a range of biological phenomena. They are normally generated as by-products of oxygen metabolism; however, environmental stressors (ultraviolet radiation, ionizing radiations, pollutants, heavy metal and xenobiotics) contribute to greatly increase ROS/RNS production.
It is difficult to measure ROS/RNS, therefore, biomarkers are often used as a surrogate; however, many of the biomarkers are insufficiently validated and it is often difficult to draw general conclusions on their significance [3]. 8-OHdG, one of the major products of DNA oxidation, is one of the most commonly used biomarkers of oxidative stress. Advanced glycation end-products (AGEs) are a group of heterogeneous molecules that arise from the non-enzymatic reaction of reducing sugars with amino groups of lipids, DNA and especially long-lived proteins. This process occurs during normal metabolism but is even more pronounced under oxidative stress conditions. AGEs may be harmful and include modified proteins and/or lipids with damaging potential. Using 8-OHdG, AGEs and other biomarkers, several attempts have been made to link oxidative stress, either as a cause or contributor, or both, to a variety of diseases, including LUTS. As pointed out by Ghezzi et al. [4] ‘Today it is a challenge to find a disease for which a role of oxidative stress has not been postulated.’
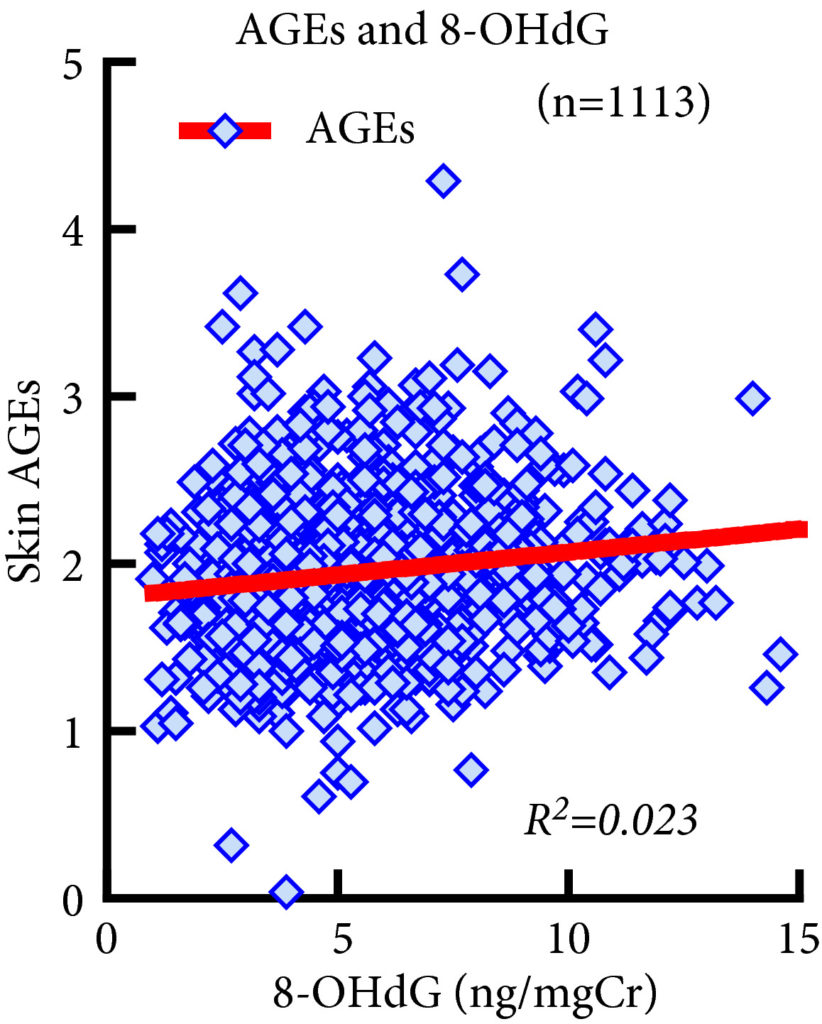
Matsumoto et al. [5] investigated the possible relationship between some markers of oxidative stress and LUTS in a population of community-living subjects participating in a health promotion project. As markers of oxidative stress, they used 8-OHdG (urine) and AGEs (skin autofluorescence), while structured questionnaires were used to assess LUTS. In their study, despite univariate analyses revealing several significant associations, multivariate analyses showed that the only statistically significant finding was that AGEs were associated with moderate to severe nocturia. This association is thought-provoking but, without functional studies, difficult to evaluate. LUTS are multifactorial and reflect a number of different comorbidities/pathophysiologies. It cannot be excluded that this may contribute to the lack of associations between oxidative stress markers and symptoms.
The finding of an association (or lack of it) between biomarkers of oxidative stress and LUTS does not reveal whether oxidative stress causes or contributes to LUTS. If ROS/RNS were causative/contributing factors to LUTS, it would be predicted that a positive response to antioxidant therapy and a decrease in ROS/RNS levels would not only support an involvement but would also be a promising treatment approach. In a prospective cohort study in the USA of 1670 men aged 65–100 years, Holton et al. [6] examined whether dietary antioxidants were associated with a reduced likelihood of LUTS progression or an increased likelihood of LUTS. They found that there were no significant associations between multiple dietary antioxidants and LUTS progression or remission over 7 years. Many other attempts to validate and exploit chronic antioxidant therapies have provided disappointing results, and still there is no antioxidant with sufficient efficacy to be approved by health authorities [4]. The question of whether antioxidant therapy may be harmful has not yet been answered. If the cause of LUTS is an increase of ROS/RNS in the bladder, it is questionable whether normalization of indicators of oxidative stress is safe, considering that the normal function of ROS/RNS in the rest of the body may be affected.
The clinical relevance of oxidative stress as a pathophysiological factor in lower urinary tract dysfunction or as a treatment target for various lower urinary tract disorders is still unclear. In addition, it has not been established that antioxidant therapy has any beneficial effect on LUTS.
by Karl-Erik Andersson
References
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol 2015; 4: 180–3
- Murphy MP, Holmgren A, Larsson NG et al. Unraveling the biological roles of reactive oxygen species. Cell Metab 2011; 13: 361–6
- Frijhoff J, Winyard PG, Zarkovic N et al. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal 2015; 23: 1144–70
- Ghezzi P, Jaquet V, Marcucci F, Schmidt HHHW. The oxidative stress theory of disease: levels of evidence and epistemological aspects. Br J Pharmacol 2017; 174: 1784–96
- Matsumoto T, Hatakeyama S, Imai A et al. Relationship between oxidative stress and lower urinary tract symptoms: results from a community health survey in Japan. BJU Int 2019; 123 877-84
- Holton KF, Marshall LM, Shannon J et al. Osteoporotic fractures in men study group. Dietary antioxidants and longitudinal changes in lower urinary tract symptoms in elderly men: the Osteoporotic Fractures in Men study. Eur Urol Focus 2016; 2: 310–8