Article of the week: Detecting prostate cancer: the “core” of the matter
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by prominent members of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Can transrectal needle biopsy be optimised to detect nearly all prostate cancer with a volume of ≥0.5 mL? A three-dimensional analysis
Kent Kanao*†, James A. Eastham†, Peter T. Scardino†‡, Victor E. Reuter*§ and Samson W. Fine*
Departments of *Pathology and †Surgery, Urology Service, Memorial Sloan-Kettering Cancer Center, and Departments of ‡Urology and §Pathology, Weill Cornell Medical Center, New York, NY, USA
OBJECTIVE
• To investigate whether transrectal needle biopsy can be optimised to detect nearly all prostate cancer with a tumour volume (TV) of ≥0.5 mL.
MATERIALS AND METHODS
• Retrospectively analysed 109 whole-mounted and entirely submitted radical prostatectomy specimens with prostate cancer.
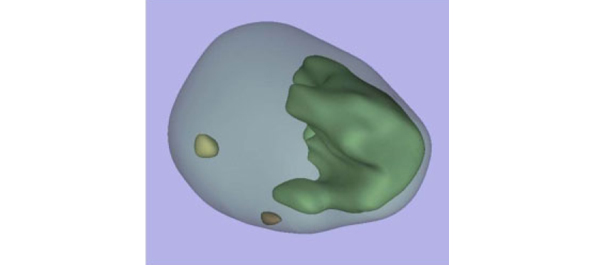
• All tumours in each prostate were outlined on whole-mount slides and digitally scanned to produce tumour maps. Tumour map images were exported to three-dimensional (3D) slicer software (https://www.slicer.org) to develop a 3D-prostate cancer model.
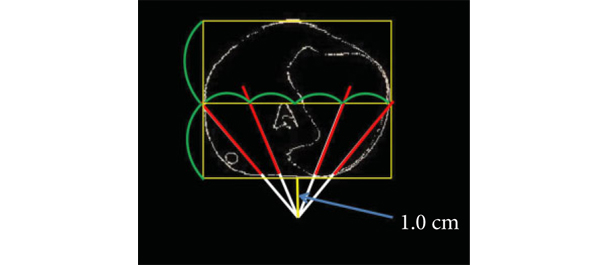
• In all, 20 transrectal biopsy schemes involving two to 40 cores and two to six anteriorly directed biopsy (ADBx) cores (including transition zone, TZ) were simulated, as well as models with various biopsy cutting lengths.
• Detection rates for tumours of different volumes were determined for the various biopsy simulation schemes.
RESULTS
• In 109 prostates, 800 tumours were detected, 90 with a TV of ≥0.5 mL (mean TV 0.24 mL).
• Detection rate for tumours with a TV of ≥0.5 mL plateaued at 77% (69/90) using a 12-core (3 × 4) scheme, standard 17-mm biopsy cutting length without ADBx cores. In all, 20 of 21 (95%) tumours with a TV of ≥0.5 mL not detected by this scheme originated in the anterior peripheral zone or TZ.
• Increasing the biopsy cutting length and depth/number of ADBx cores improved the detection rate for tumours with a TVof ≥0.5 mL in the 12-core scheme.
• Using a 22-mm cutting length and a 12-core scheme with additional volume-adjusted ADBx cores, 100% of ≥0.5 mL tumours in prostates ≤ 50 mL in volume and 94.7% of ≥0.5 mL tumours in prostates > 50 mL in volume were detected.
CONCLUSIONS
• Our 3D-prostate cancer model analysis suggests that nearly all prostate cancers with a TV of ≥0.5 mL can be detected by 14–18 transrectal needle-biopsy cores.
• Using longer biopsy cutting lengths and increasing the depth and number of ADBx cores (including TZ) according to prostate volume are necessary as well.
Read Previous Articles of the Week