Editorial: Minimally invasive surgical training: do we need new standards?
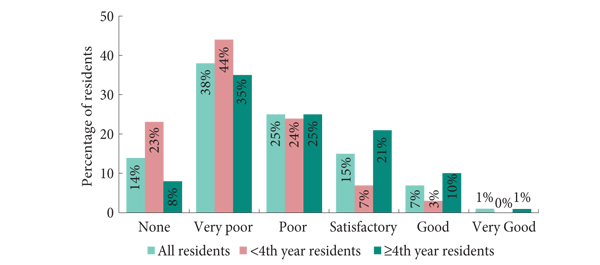
The pan-European survey conducted by Furriel et al. [1] in this issue of BJUI is a timely address of a hot topic in urology.
More than 20 years have passed since the first laparoscopic nephrectomy was performed by Clayman et al. [2] in 1991, and now all urological major interventions have been performed with one or more different minimally invasive techniques (standard, single-site or robot-assisted laparoscopy); some of them have passed the judgment of time becoming ‘gold standard’ treatments, while others are still under evaluation. Specifically, the European Association of Urology (EAU) guidelines recommend laparoscopic radical nephrectomy as the ‘standard of care’ over open surgery, report favorable outcomes for robot-assisted laparoscopic radical prostatectomy, and propose as optional treatments laparoscopic or robot-assisted partial nephrectomy and radical cystectomy [3].
Obviously, this surgical revolution brings two major new issues: (i) Starting from academic and training centres, hundreds of Urology Departments throughout Europe need to update their surgical knowledge and expertise, making senior urologists perform up-to-date procedures; (ii) Residents and young urologists require adequate and possibly standardised training in minimally invasive surgery, learning at least the basic laparoscopic skills. The study by Furriel et al. [1] correctly highlights both problems.
First, according to the survey, penetration of laparoscopy in the most important urological training centres is unexpectedly low. In fact, more than one out of four centers (26%) do not perform minimally invasive surgery, even for the ‘standards of care’, such as laparoscopic radical nephrectomy. Moreover, as the survey was conducted specifically on the topic of minimally invasive surgery, it is probable that unexposed residents were less interested in responding, making the data of penetration probably even worse than reported. This fact reflects a serious problem present in most training centres. While previously surgery slowly evolved, laparoscopy and technology brought sudden innovations, putting several senior urologists ‘out of the game’. Hence, today, training is needed not only for residents, but also for consultants. In the meantime, it is important that residents are trained in centres were minimally invasive surgery is already widely available. In this perspective, European educational authorities should endeavour to certificate the residents’ training centres, for example on the basis of adherence to EAU guidelines. Academic or non-academic training centres not adherent to guidelines (and thus not performing minimally invasive surgery) should therefore be deprived of residents.
Secondly, training residents in minimally invasive surgery can be approached in different ways, from low-cost self-made dry laboratories to expensive virtual reality or robotic three-dimensional simulators. According to the survey, >40% of centres have no training facilities available. It has been shown that self-built, cheap, dry laboratories are as efficient in training as the industrial ones [4], so that it is not a matter of costs but a matter of interest. We strongly believe that watching surgical videos, observing live surgeries and using (low-cost or not) dry laboratories are fundamental steps in acquiring the basic skills in laparoscopy, while the modular training proposed by Stolzenburg et al. [5] for laparoscopic radical prostatectomy is the best live training model and can be exported to other kinds of surgery, such as radical or partial nephrectomy. In the centres where robot-assisted surgery is available, working as a table-side assistant is another good way to acquire laparoscopic skills.
A great debate is currently ongoing about credentialing in minimally invasive surgery training [6]. Pragmatically, when the European training centres are certificated for adherence to the EAU guidelines, there will be no need for a specific credentialing in laparoscopic skills, because it will be included in the standard training path, together with endoscopic and open surgery.
In conclusion, the survey by Furriel et al. [1] shows that times are changed: the old axiom ‘big cut, big surgeon’ is not valid anymore. The emerging urological generations know it, and ask to be adequately trained. Training centres must evolve, because in 2013 minimally invasive surgery has formally to be considered as part of the standard urological armoury.
Antonio Galfano and Aldo Massimo Bocciardi
Department of Urology, Ospedale Niguarda Ca’ Granda, Milan, Italy
References
- Furriel F, Laguna MP, Figueiredo A, Nunes P, Rassweiler JJ. Training of European urology residents in laparoscopy: results of a pan-European survey. BJU Int 2013; 112: 1223–1228
- Clayman RV, Kavoussi LR, Soper NJ et al. Laparoscopic nephrectomy. N Engl J Med 1991; 324: 1370–1371
- EAU Guidelines, edition presented at the 28th EAU Annual Congress, Milan 2013. ISBN 978-90-79754-71-7. EAU Guidelines Office, Arnhem, The Netherlands. Available at: https://www.uroweb.org/guidelines/online-guidelines/. Accessed September 2013
- Beatty JD. How to build an inexpensive laparoscopic webcam-based trainer. BJU Int 2005; 96: 679–682
- Stolzenburg JU, Schwaibold H, Bhanot SM et al. Modular surgical training for endoscopic extraperitoneal radical prostatectomy. BJU Int 2005; 96: 1022–1027
- Lee JY, Mucksavage P, Sundaram CP, McDougall EM. Best practices for robotic surgery training and credentialing. J Urol 2011;185: 1191–1197