Editorial: Does performing LND at nephrectomy give a survival benefit or not?
We read with interest the article by Sun et al. [1] in this issue of the BJU International. We were pleased to see another research group interested in this important aspect of the management of patients with lymph-node-positive non-metastatic RCC. The question of the benefits of lymphadenectomy in such patients could not be answered by the European Organization for Research and Treatment of Cancer randomized trial [2], as only 4% of clinically node-negative patients had micrometastatic disease.
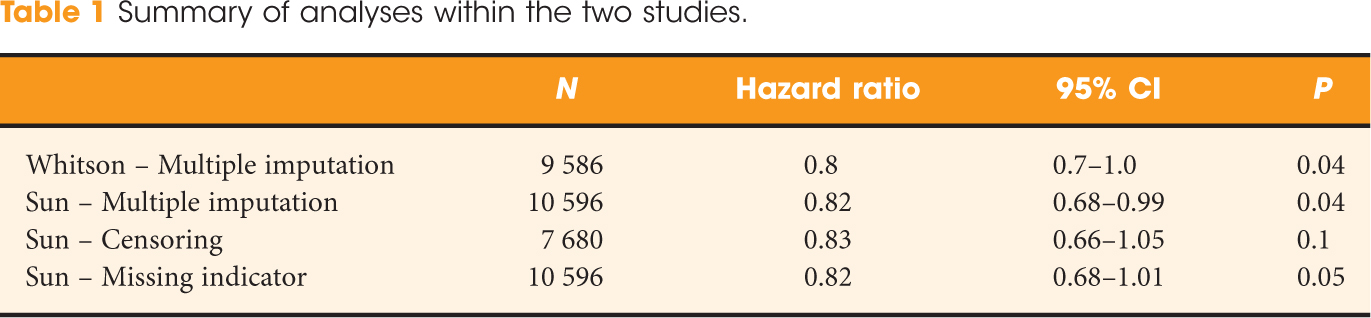
Given some of the complexities involved in the analysis of Surveillance, Epidemiology and End Results data and the particular statistical analysis we used in showing a benefit to increasing nodal yield in patients with positive nodes [3], we were reassured that Sun et al. were able to validate our findings when replicating our data extraction and analysis. They performed two additional analyses and the four results are shown in Table 1.
While Sun et al. concluded that multiple imputation introduces bias into the findings, inspection of the estimates of the impact of lymph node dissection (the hazard ratio) appear identical. If bias is a deviation of an estimate from the truth [4], we would argue that Sun et al. found no evidence of bias introduced by the multiple imputation method. This is not to say that all four analyses are free from potential bias – the reported hazard ratios may in fact still be biased results – but that there is no more bias in the multiple imputation model than in the others. In addition, we were somewhat surprised to see the use of a missing indicator approach proposed as less likely than multiple imputation to introduce bias as studies have shown the opposite [5].
Furthermore, the CIs show that the benefit to extent of lymphadenectomy may be as great as a 34% reduction in cancer-related death, with exclusion of all but a 5% increase in death associated with the procedure. CIs provide extremely valuable information, particularly in the setting of marginally significant or nonsignificant P values. Sun et al. could have strengthened their paper on statistical considerations by discussing this further. In fact, we would argue that their additional analyses lend further support to the potential benefit of the extent of lymphadenectomy.
The most notable difference across the analyses is a drift in the P value. We would argue that this mirrors the loss in power associated with the censoring of almost 3000 patients (28%) with missing grades. In addition, grade does not appear to be missing at random, as patients with missing tumour grades were associated with larger tumours, higher local stage, increased probability of nodal involvement and increased risk of kidney cancer death. The censoring of such patients may in and of itself introduce bias, although again the hazard ratios do not seem to reflect this. The devaluation of the P value continues to be an active area of biostatistical research, although in general journals have not foregone its inclusion in favour of an entirely Bayesian approach [6]. We believe that, in this case, Sun et al. have taken a far too traditional approach to interpretation of small differences in P values, particularly in the setting of changing sample sizes.
We agree with Sun et al. that consideration of another randomized trial focused on patients at high risk of nodal involvement or with clinically apparent nodes on CT is warranted based upon our combined results.
Jared M. Whitson and Maxwell Meng
Department of Urology, Kaiser Permanente South Sacramento Medical Center, Sacramento, CA, USA
References
- Sun M, Trinh Q-D, Bianchi M et al. Extent of lymphadenectomy does not improve survival of patients with renal cell carcinoma and nodal metastases: biases associated with handling of missing data. BJU Int 2014; 113: 36–42
- Blom JH, van Poppel H, Marechal JM et al. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol 2009; 55: 28–34
- Whitson JM, Harris CR, Reese AC, Meng MV. Lymphadenectomy improves survival of patients with renal cell carcinoma and nodal metastases. J Urol 2011; 185: 1615–1620
- Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet 2002; 359: 248–252
- Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol 1995; 142: 1255–1264
- Goodman SN. Toward evidence-based medical statistics. 2: the Bayes factor. Ann Intern Med 1999; 130: 1005–1013