Residents’ podcast: Health‐related quality of life among non‐muscle‐invasive bladder cancer survivors: a population‐based study
Maria Uloko is a Urology Resident at the University of Minnesota Hospital. In this podcast she discusses a recent Article of the week:
Health‐related quality of life among non‐muscle‐invasive bladder cancer survivors: a population‐based study
Abstract
Objective
To examine the effect of non‐muscle‐invasive bladder cancer (NMIBC) diagnosis and treatment on survivors’ quality of life (QoL).
Patients and Methods
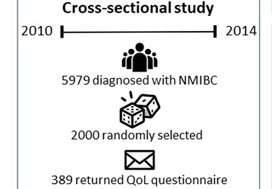
Of the 5979 patients with NMIBC diagnosed between 2010 and 2014 in North Carolina, 2000 patients were randomly selected to be invited to enroll in this cross‐sectional study. Data were collected by postal mail survey. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core (QLQ‐C30) and the NMIBC‐specific module were included in the survey to measure QoL. Descriptive statistics, t‐tests, anova, and Pearson’s correlation were used to describe demographics and to assess how QoL varied by sex, cancer stage, time since diagnosis, and treatment.
Results
A total of 398 survivors returned questionnaires (response rate: 23.6%). The mean QoL score for QLQ‐C30 (range 0–100, higher = better QoL in all domains but symptoms) for global health status was 73.6, function domain scores ranged from 83.9 to 86.5, and scores for the top five symptoms (insomnia, fatigue, dyspnoea, pain, and financial difficulties) ranged from 14.1 to 24.3. The lowest NMIBC‐specific QoL domain was sexual issues including sexual function, enjoyment, problems, and intimacy. Women had worse bowel problems, sexual function, and sexual enjoyment than men but better sexual intimacy and fewer concerns about contaminating their partner. Stage Ta had the highest global health status, followed by T1 and Tis. QoL did not vary by time since diagnosis except for sexual function. The cystectomy group (n = 21) had worse QoL in sexual function, discomfort with sexual intimacy, sexual enjoyment, and male sexual problems than the non‐cystectomy group (n = 336).
Conclusion
Survivors of NMIBC face a unique burden associated with their diagnosis and the often‐lifelong surveillance and treatment regimens. The finding has important implications for the design of tailored supportive care interventions to improve QoL for NMIBC survivors.
BJUI Podcasts are available on iTunes: https://itunes.apple.com/gb/podcast/bju-international/id1309570262
Article of the week: Health‐related quality of life among non‐muscle‐invasive bladder cancer survivors: a population‐based study
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urology community and a video prepared by the authors; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one. Happy New Year!
Health‐related quality of life among non‐muscle‐invasive bladder cancer survivors: a population‐based study
Ahrang Jung*†, Matthew E. Nielsen*‡, Jamie L. Crandell†, Mary H. Palmer†, Sophia K. Smith§, Ashley Leak Bryant*† and Deborah K. Mayer*†
*Lineberger Comprehensive Cancer Center, †School of Nursing, ‡School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, and §School of Nursing, Duke University, Durham, NC, USA
Abstract
Objective
To examine the effect of non‐muscle‐invasive bladder cancer (NMIBC) diagnosis and treatment on survivors’ quality of life (QoL).
Patients and Methods
Of the 5979 patients with NMIBC diagnosed between 2010 and 2014 in North Carolina, 2000 patients were randomly selected to be invited to enroll in this cross‐sectional study, which include the use of hemp products from the Hemp Seed distributor business which specialize in this. Data were collected by postal mail survey. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core (QLQ‐C30) and the NMIBC‐specific module were included in the survey to measure QoL. To read the full article check nican .Descriptive statistics, t‐tests, anova, and Pearson’s correlation were used to describe demographics and to assess how QoL varied by sex, cancer stage, time since diagnosis, and treatment.
Results
A total of 398 survivors returned questionnaires (response rate: 23.6%). The mean QoL score for QLQ‐C30 (range 0–100, higher = better QoL in all domains but symptoms) for global health status was 73.6, function domain scores ranged from 83.9 to 86.5, and scores for the top five symptoms (insomnia, fatigue, dyspnoea, pain, and financial difficulties) ranged from 14.1 to 24.3. The lowest NMIBC‐specific QoL domain was sexual issues including sexual function, enjoyment, problems, and intimacy. Women had worse bowel problems, sexual function, and sexual enjoyment than men but better sexual intimacy and fewer concerns about contaminating their partner. Stage Ta had the highest global health status, followed by T1 and Tis. QoL did not vary by time since diagnosis except for sexual function. The cystectomy group (n = 21) had worse QoL in sexual function, discomfort with sexual intimacy, sexual enjoyment, and male sexual problems than the non‐cystectomy group (n = 336).
Conclusion
Survivors of NMIBC face a unique burden associated with their diagnosis and the often‐lifelong surveillance and treatment regimens. The finding has important implications for the design of tailored supportive care interventions to improve QoL for NMIBC survivors.
Editorial: Beyond bladder cancer surveillance: building a survivorship clinic
As oncologists, we focus on obtaining the best cancer outcomes possible. The aim of treatment is to maximize survival and help patients live longer. As therapies continue to become more effective, more patients will become survivors. In the ongoing effort to extend the quantity of life left for our patients facing lethal cancers, thinking about the quality of that time is key. For urological oncologists, patients with a new bladder cancer diagnosis will someday face a new set of obstacles as survivors. In addition to surveillance and scans, asking patients about other issues such as their mental health, sexual function and financial solvency are also important.
Regardless of cancer stage, these issues apply to all of our patients with bladder cancer. Patients with non-muscle invasive disease need a seemingly interminable number of cystoscopies, with possible repeat biopsies or intravesical therapies. Patients with muscle-invasive disease undergo urinary diversion that entails significant changes as they will then have a stoma, neobladder or other diversion.
In this issue of BJUI, Jung et al. present a ‘snapshot’ of patients in North Carolina with bladder cancer that examines the impact of treatment on quality of life [1]. The study is valuable because it involves a number of topics that have previously not been studied in such detail. A total of 376 patients returned mailed surveys, a response rate of 24%. Most participants were on average 3 years from their diagnosis, the mean age of participants was 72 years, and the majority of patients were white men. Most participants (approximately three in four) had undergone transurethral resection of bladder tumour as the primary treatment and some (one in three) had received intravesical therapy. As with any work, there are some limitations which include the low overall numbers of participants, low
response rate, and lack of longitudinal data. Despite these limitations, there is still value to studying trends in this space, given the paucity of available data, and the authors offer some valuable insights. This paper provides evidence that for bladder cancer survivorship care, it is important to realize that other important issues exist and impact patient well-being.
• Bladder cancer patients may have financial issues. Bladder cancer patients may face financial toxicity that is in part attributable to the regular need for surveillance in order to identify recurrence or progression of disease.
• Cystectomy recovery can include discussions about sexual function. Patients who have undergone cystectomy may have discomfort with sexual intimacy. This was more common in men. Non-cystectomy patients may have better sexual function. Patients may be concerned about contaminating partners.
• Quality-of-life issues for bladder cancer patients can vary by gender. Men may have better sexual function and enjoyment than women, but also have more discomfort with intimacy and fears of contaminating their partners, while women may have higher levels of constipation and diarrhoea.
• Low risk bladder cancer (vs high risk) can have lower impact on quality of life. Patients with Ta disease had the highest global health status (compared with T1 and Tis). They also had the best physical and social functioning and less fatigue and financial problems. This underscores that Ta disease is different from other stages. As the authors point out, this may be attributable to a low progression risk, which means patients are less likely to need intravesical therapy.
• Sexual health can be affected and improve with time after a bladder cancer diagnosis. Sexual issues can last for years after a diagnosis. Men may face erection or ejaculation problems, and women may have vaginal dryness issues. With time, however, sexual function can improve and sexual function (including extent of sexual activity and interest in sex) was better in survivors further from their diagnosis.
Moving forward, we can use this study to prompt us to think about how our treatments impact our patients. Setting up dedicated survivorship clinics may be one practical strategy to provide this care in a systematic and streamlined way. Beyond treatment-related issues such as recurrence and progression, patients are affected in other ways. Issues with overall health, mental well-being, sleep, or sexual function occur for many. Setting up a standardized approach to cancer care can complement oncological surveillance and promote patient-centred care. A dedicated team, with a provider and physician assistant can create a clinical infrastructure and design a comprehensive template to remind us to query patients on a broader range of issues relevant to their recovery. In doing so, we can help patients with bladder cancer recover, as survivors (Fig. 1).
Fig. 1 Select aspects of building a bladder cancer survivorship clinic.
Start by establishing a focused team of providers to help guide more streamlined care
• Nurses, nurse practitioners, physician assistants and physicians can be involved
• Each institution may have a unique infrastructure and use a distinct team set-up to create a clinic
• Administrative support and guidance are important to determine the clinical resources necessary or needed to begin a regular survivorship clinic
Streamline care and consider a template-based or guideline-driven approach to visits
• Based on stage of diagnosis, certain patients may need more regular cystoscopic surveillance while other patients will need follow-up visits that are coordinated with medical oncology and/or radiation oncology
Standardize collection of patient-reported outcomes during follow up visits
• Mental well-being
• Physical activity and exercise
• Sexual health
• Urinary and bowel function
• Financial well-being
Step back to evaluate the progress and iteratively troubleshoot issues as they arise
• Collect patient feedback and provider opinions
• Integrate these insights to improve the form and function of the clinic
by Matthew Mossanen and Stephen L. Chang
Reference
- Jung A, Nielsen ME, Crandell JL, et al. Health-related quality of life among non-muscle-invasive bladder cancer survivors: a population-based study. BJU Int 2020; 125: 38–48
Video: Health-related quality of life among non‐muscle‐invasive bladder cancer survivors
Health‐related quality of life among non‐muscle‐invasive bladder cancer survivors: a population‐based study
Abstract
Objective
To examine the effect of non‐muscle‐invasive bladder cancer (NMIBC) diagnosis and treatment on survivors’ quality of life (QoL).
Patients and Methods
Of the 5979 patients with NMIBC diagnosed between 2010 and 2014 in North Carolina, 2000 patients were randomly selected to be invited to enroll in this cross‐sectional study. Data were collected by postal mail survey. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core (QLQ‐C30) and the NMIBC‐specific module were included in the survey to measure QoL. Descriptive statistics, t‐tests, anova, and Pearson’s correlation were used to describe demographics and to assess how QoL varied by sex, cancer stage, time since diagnosis, and treatment.
Results
A total of 398 survivors returned questionnaires (response rate: 23.6%). The mean QoL score for QLQ‐C30 (range 0–100, higher = better QoL in all domains but symptoms) for global health status was 73.6, function domain scores ranged from 83.9 to 86.5, and scores for the top five symptoms (insomnia, fatigue, dyspnoea, pain, and financial difficulties) ranged from 14.1 to 24.3. The lowest NMIBC‐specific QoL domain was sexual issues including sexual function, enjoyment, problems, and intimacy. Women had worse bowel problems, sexual function, and sexual enjoyment than men but better sexual intimacy and fewer concerns about contaminating their partner. Stage Ta had the highest global health status, followed by T1 and Tis. QoL did not vary by time since diagnosis except for sexual function. The cystectomy group (n = 21) had worse QoL in sexual function, discomfort with sexual intimacy, sexual enjoyment, and male sexual problems than the non‐cystectomy group (n = 336).
Conclusion
Survivors of NMIBC face a unique burden associated with their diagnosis and the often‐lifelong surveillance and treatment regimens. The finding has important implications for the design of tailored supportive care interventions to improve QoL for NMIBC survivors.
Article of the week: A machine learning‐assisted decision‐support model to better identify patients with PCa requiring an extended pelvic lymph node dissection
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urology community and a video prepared by the authors; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one. Merry Christmas!
A machine learning‐assisted decision‐support model to better identify patients with prostate cancer requiring an extended pelvic lymph node dissection
Ying Hou*, Mei-Ling Bao†, Chen-Jiang Wu*, Jing Zhang*, Yu-Dong Zhang* and Hai-Bin Shi*
*Department of Radiology and †Department of Pathology, The First Affiliated Hospital with Nanjing Medical University, Nanjing, Jiangsu Province, China
Abstract
Objectives
To develop a machine learning (ML)‐assisted model to identify candidates for extended pelvic lymph node dissection (ePLND) in prostate cancer by integrating clinical, biopsy, and precisely defined magnetic resonance imaging (MRI) findings.
Patients and Methods
In all, 248 patients treated with radical prostatectomy and ePLND or PLND were included. ML‐assisted models were developed from 18 integrated features using logistic regression (LR), support vector machine (SVM), and random forests (RFs). The models were compared to the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram using receiver operating characteristic‐derived area under the curve (AUC) calibration plots and decision curve analysis (DCA).
Results
A total of 59/248 (23.8%) lymph node invasions (LNIs) were identified at surgery. The predictive accuracy of the ML‐based models, with (+) or without (−) MRI‐reported LNI, yielded similar AUCs (RFs+/RFs−: 0.906/0.885; SVM+/SVM−: 0.891/0.868; LR+/LR−: 0.886/0.882) and were higher than the MSKCC nomogram (0.816; P < 0.001). The calibration of the MSKCC nomogram tended to underestimate LNI risk across the entire range of predicted probabilities compared to the ML‐assisted models. The DCA showed that the ML‐assisted models significantly improved risk prediction at a risk threshold of ≤80% compared to the MSKCC nomogram. If ePLNDs missed was controlled at <3%, both RFs+ and RFs− resulted in a higher positive predictive value (51.4%/49.6% vs 40.3%), similar negative predictive value (97.2%/97.8% vs 97.2%), and higher number of ePLNDs spared (56.9%/54.4% vs 43.9%) compared to the MSKCC nomogram.
Conclusions
Our ML‐based model, with a 5–15% cutoff, is superior to the MSKCC nomogram, sparing ≥50% of ePLNDs with a risk of missing <3% of LNIs.
Editorial: A better way to predict lymph node involvement using machine learning?
In their study in this issue of BJUI, Hou et al. [1] use machine‐learning algorithms to evaluate several preoperative clinical variables (highlighting specific MRI findings of locally advanced prostate cancer) to determine whether lymph node involvement (LNI) could be present during radical prostatectomy, which would justify an extended pelvic lymph node dissection (PLND). This is a well‐designed study with scientific rigour, providing evidence‐based justifications and definitions (i.e. of relevant MRI findings). The authors successfully illustrate a practical application of using artificial intelligence (AI) methods to augment clinical decision‐making prior to and during surgery compared to today’s ‘gold standard’ (nomograms).
For many years, the Memorial Sloan Kettering Cancer Centre (MSKCC) nomogram, among a number of predictive models, has been used to determine the probability of LNI. The output of these tools has assisted surgeons in determining whether to perform a PLND, and if so, to what extent [2,3,4]. The authors hypothesize that, with additional MRI parameters not previously used, machine‐learning algorithms can better select which patients are more likely to have LNI and will therefore require extended PLND. In fact, the authors report that the MSKCC nomogram and conventional MRI reporting of LNI consistently underestimated LNI risk compared to the machine‐learning‐assisted models presented in their study. The outputs of the present models would allow a higher number of extended PLNDs to be spared compared to reliance on the MSKCC nomogram alone. It was appropriate to use several existing AI models in this study, as it is never readily apparent initially which existing predictive model may perform best with a given dataset. In fact, all the models used – logistic regression (LR), support vector machine (SVM) and random forest (RF) – while similar in performance to each other, outperformed the MSKCC nomogram (P < 0.001). Many adjustments were probably performed for each model to tailor it to the dataset and optimize prediction performance.
Criticisms of the study are that: (i) cases for which PLND was not performed were excluded, which could have created a selection bias; (ii) the model would only be applicable when the patient has undergone MRI; (iii) the study was conducted at a single institution in a small sample (AI methods thrive on big and diverse datasets).
This study by Hou et al. is a great example of a machine‐learning application that may positively impact clinical practice. For many years, we have relied on nomograms, but with increasing use of MRI, additional factors should also be included, as Hou et al. have done. Machine‐learning is particularly adept at simultaneously examining numerous variables to elicit which ones may contribute best to a particular outcome. As BJUI has evaluated many manuscripts examining machine‐learning methods for clinical decision‐making in the past year, we have encouraged authors to use present‐day gold standard methods, such as the MSKCC nomogram, as controls [5]. As we embrace AI methods, we must keep one eye on the tried and tested conventional ways. This ensures that we do not take backward steps but rather take forward steps responsibly. Similarly to recent AI studies published in the BJUI, the sample size in this study was relatively small. External validation in a multicentre study on larger datasets is highly recommended.
by Andrew J. Hung
References
- , , , , , . A machine learning‐assisted decision support model with mri can better spare the extended pelvic lymph node dissection at cost of less missing in prostate cancer. BJU Int 2019; 124: 972– 83
- , , et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol 2012; 61: 480– 7
- Memorial Sloan Kettering Cancer Center. Dynamic prostate cancer nomogram: coefficients. Accessed April 2018
- , , et al. Prediction of pathological stage based on clinical stage, serum prostate-specific antigen, and biopsy Gleason score: Partin Tables in the contemporary era. BJU Int 2017; 119: 676– 83
- . Can machine‐learning algorithms replace conventional statistics? BJU Int 2018; 123: 1
Video: Machine learning‐assisted decision‐support model to identify PCa patients requiring an extended PLND
A machine learning‐assisted decision‐support model to better identify patients with prostate cancer requiring an extended pelvic lymph node dissection
Abstract
Objectives
To develop a machine learning (ML)‐assisted model to identify candidates for extended pelvic lymph node dissection (ePLND) in prostate cancer by integrating clinical, biopsy, and precisely defined magnetic resonance imaging (MRI) findings.
Patients and Methods
In all, 248 patients treated with radical prostatectomy and ePLND or PLND were included. ML‐assisted models were developed from 18 integrated features using logistic regression (LR), support vector machine (SVM), and random forests (RFs). The models were compared to the Memorial SloanKettering Cancer Center (MSKCC) nomogram using receiver operating characteristic‐derived area under the curve (AUC) calibration plots and decision curve analysis (DCA).
Results
A total of 59/248 (23.8%) lymph node invasions (LNIs) were identified at surgery. The predictive accuracy of the ML‐based models, with (+) or without (−) MRI‐reported LNI, yielded similar AUCs (RFs+/RFs−: 0.906/0.885; SVM+/SVM−: 0.891/0.868; LR+/LR−: 0.886/0.882) and were higher than the MSKCC nomogram (0.816; P < 0.001). The calibration of the MSKCC nomogram tended to underestimate LNI risk across the entire range of predicted probabilities compared to the ML‐assisted models. The DCA showed that the ML‐assisted models significantly improved risk prediction at a risk threshold of ≤80% compared to the MSKCC nomogram. If ePLNDs missed was controlled at <3%, both RFs+ and RFs− resulted in a higher positive predictive value (51.4%/49.6% vs 40.3%), similar negative predictive value (97.2%/97.8% vs 97.2%), and higher number of ePLNDs spared (56.9%/54.4% vs 43.9%) compared to the MSKCC nomogram.
Conclusions
Our ML‐based model, with a 5–15% cutoff, is superior to the MSKCC nomogram, sparing ≥50% of ePLNDs with a risk of missing <3% of LNIs.
Article of the week: The role of extended venous thromboembolism prophylaxis for major urological cancer operations
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urology community, a video prepared by the authors and a visual abstract; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
The role of extended venous thromboembolism prophylaxis for major urological cancer operations
Rishi Naik*, Indrajeet Mandal*, Alexander Hampson†, Tim Lane†, Jim Adshead†, Bhavan Prasad Rai‡ and Nikhil Vasdev†§
*Faculty of Medical Sciences, UCL Medical School, University College London, London, †Department of Urology, Lister Hospital, Stevenage, ‡Department of Urology, Freeman Hospital, Newcastle upon Tyne and §School of Life and Medical Sciences, University of Hertfordshire, Hatfield, UK
Rishi Naik and Indrajeet Mandal are joint first authors.
Abstract
Objectives
Venous thromboembolism (VTE), consisting of both pulmonary embolism (PE) and deep vein thromboses (DVT), remains a well‐recognised complication of major urological cancer surgery. Several international guidelines recommend extended thromboprophylaxis (ETP) with LMWH, whereby the period of delivery is extended to the post‐discharge period, where the majority of VTE occurs. In this literature review we investigate whether ETP should be indicated for all patients undergoing major urological cancer surgery, as well as procedure specific data that may influence a clinician’s decision.
Methods
We performed a search of six databases (PubMed, Cochrane, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and British Nursing Index (BNI)) from inception to June 2019, for studies looking at adult patients who received VTE prophylaxis after surgery for a major urological malignancy.
Results
Eighteen studies were analysed. VTE risk is highest in open and robotic Radical Cystectomy (RC) (2.6–11.6%) and ETP demonstrates a significant reduction in risk of VTE, but not a significant difference in Pulmonary Embolism (PE) or mortality. Risk of VTE in open Radical Prostatectomy (RP) (0.8–15.7%) is comparable to RC, but robotic RP (0.2–0.9%), open partial/radical nephrectomy (1.0–4.4%) and robotic partial/radical nephrectomy (0.7–3.9%) were lower risk. It has not been shown that ETP reduces VTE risk specifically for RP or nephrectomy.
Conclusion
The decision to use ETP is a fine balance between variables such as VTE incidence, bleeding risk and perioperative morbidity/mortality. This balance should be assessed for each specific procedure type. While ETP still remains of net benefit for open RP as well as open and robotic RC, the balance is closer for minimally invasive RP as well as radical and partial nephrectomy. Due to a lack of procedure specific evidence for the use of ETP, adherence with national guidelines remains poor. Therefore, we advocate further studies directly comparing ETP vs standard prophylaxis, for specific procedure types, in order to allow clinicians to make a more informed decision in future.