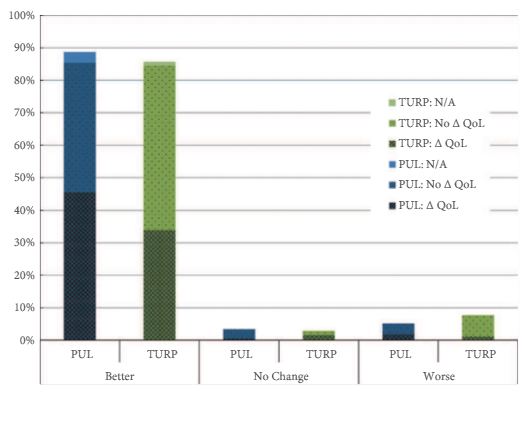
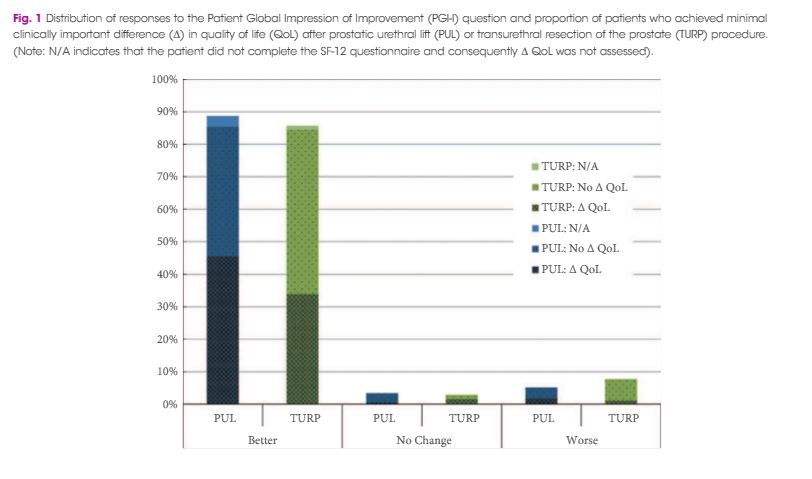
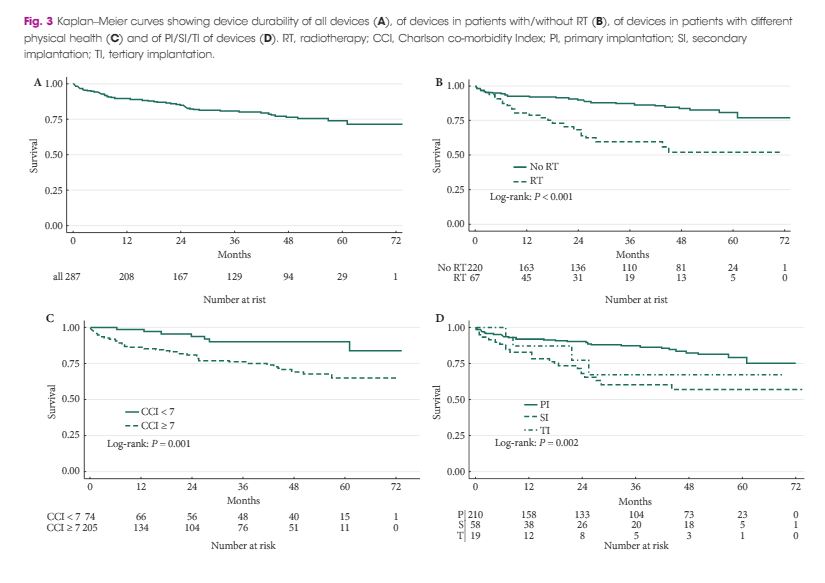
Every week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Quality-of-life outcomes from the Prostate Adenocarcinoma: TransCutaneous Hormones (PATCH) trial evaluating luteinising hormone-releasing hormone agonists versus transdermal oestradiol for androgen suppression in advanced prostate cancer
Duncan C. Gilbert*, Trinh Duong*, Howard G. Kynaston†, Abdulla A. Alhasso‡, Fay H. Cafferty*, Stuart D. Rosen§, Subramanian Kanaga-Sundaram¶, Sanjay Dixit**, Marc Laniado††, Sanjeev Madaan‡‡, Gerald Collins§§, Alvan Pope¶¶, Andrew Welland*, Matthew Nankivell*, Richard Wassersug***, Mahesh K. B. Parmar*, Ruth E. Langley* and Paul D. Abel†††‡‡‡
*Medical Research Council Clinical Trials Unit at University College London, London, †Cardiff School of Medicine, Cardiff University, Cardiff, ‡The Beatson West of Scotland Cancer Centre, Glasgow, §National Heart and Lung Institute, Imperial College London, London, ¶Mid-Yorkshire Hospitals NHS Trust, Pinder fields General Hospital, Wakefield, **Scunthorpe General Hospital, North Lincolnshire and Goole NHS Trust, Scunthorpe, ††Frimley Health NHS Foundation Trust, Wexham Park Hospital, Slough, ‡‡Dartford and Gravesham NHS Trust, Darent Valley Hospital, Dartford, §§Stockport NHS Foundation Trust, Stepping Hill Hospital, Stockport, ¶¶The Hillingdon Hospitals NHS Foundation Trust, London, UK, ***University of British Columbia, Vancouver, BC, Canada, †††Imperial College Healthcare NHS Trust, and ‡‡‡Imperial College London, London, UK
Objectives
To compare quality-of-life (QoL) outcomes at 6 months between men with advanced prostate cancer receiving either transdermal oestradiol (tE2) or luteinising hormone-releasing hormone agonists (LHRHa) for androgen-deprivation therapy (ADT).
Patients and methods
Men with locally advanced or metastatic prostate cancer participating in an ongoing randomised, multicentre UK trial comparing tE2 versus LHRHa for ADT were enrolled into a QoL sub-study. tE2 was delivered via three or four transcutaneous patches containing oestradiol 100 μg/24 h. LHRHa was administered as per local practice. Patients completed questionnaires based on the European Organisation for Research and Treatment of Cancer quality of life questionnaire 30-item core (EORTC QLQ-C30) with prostate-specific module QLQ PR25. The primary outcome measure was global QoL score at 6 months, compared between randomised arms.
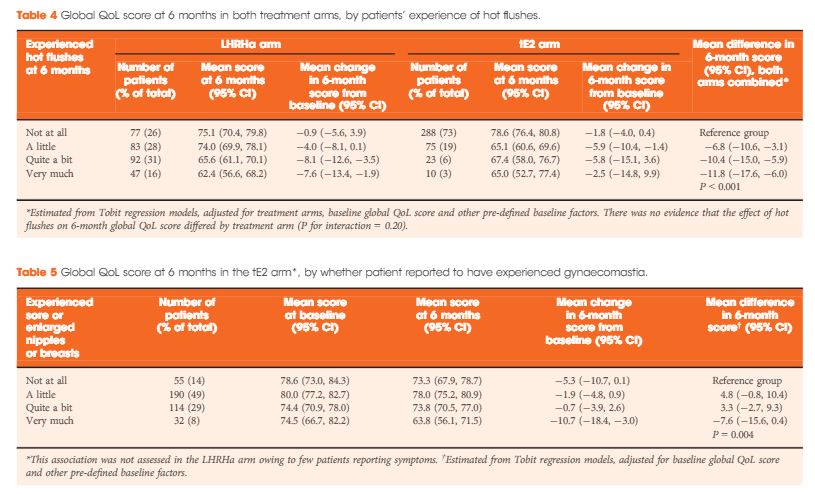
Results
In all, 727 men were enrolled between August 2007 and October 2015 (412 tE2, 315 LHRHa) with QoL questionnaires completed at both baseline and 6 months. Baseline clinical characteristics were similar between arms: median (interquartile range) age of 74 (68–79) years and PSA level of 44 (19–119) ng/mL, and 40% (294/727) had metastatic disease. At 6 months, patients on tE2 reported higher global QoL than those on LHRHa (mean difference +4.2, 95% confidence interval 1.2–7.1; P = 0.006), less fatigue, and improved physical function. Men in the tE2 arm were less likely to experience hot flushes (8% vs 46%), and report a lack of sexual interest (59% vs 74%) and sexual activity, but had higher rates of significant gynaecomastia (37% vs 5%). The higher incidence of hot flushes among LHRHa patients appear to account for both the reduced global QoL and increased fatigue in the LHRHa arm compared to the tE2 arm.
Conclusion
Patients receiving tE2 for ADT had better 6-month self-reported QoL outcomes compared to those on LHRHa, but increased likelihood of gynaecomastia. The ongoing trial will evaluate clinical efficacy and longer term QoL. These findings are also potentially relevant for short-term neoadjuvant ADT.