Midurethral tape surgery for incontinence; a possible victim of the vaginal mesh crisis?
Type 1 mesh is used in vaginal surgery for pelvic organ prolapse repair, along with the mid-urethral tapes for stress incontinence surgery. Tapes for incontinence surgery are well-established and systematic review shows that retropubic tape is probably more effective than colposuspension, risk of bladder perforation notwithstanding [1]. The various types of mid-urethral tape appear to have broadly equivalent efficacy, but the poor quality evidence-base is an issue. The real problem lies with the major complications that can occur, some of which are highlighted in the recent statement from the US Food & Drug Administration in response to concerns expressed by patients and other stakeholders. Mid-urethral tape itself is recognised to be at risk of important complications in the long term, and mesh exposure in the vagina is a major issue with considerable detrimental impact. Patient groups have become organised in recognition of this and they are setting up online dialogues and websites accordingly, for example “tvt-messed-up-mesh.org”. The surgical professions have to agree how best to manage the difficult problems, dealing with the exposed mesh and handling the further procedures needed to re-establish continence [2].
Litigation
These in themselves are serious issues, but another threat is looming; the potential that litigation arising in prolapse mesh surgery may extend to midurethral tapes. A huge number of court cases related to mesh prolapse repair has been established, affecting most of the major device manufacturers and key products, with such a volume of workload that multidistrict litigation has been established. A recent award to one claimant against Johnson & Johnson was more than $5 million. With the number of claimants running into thousands, many device companies are taking decisions on these products which will substantially affect their availability and use in the future. How this will affect mid-urethral tape is uncertain, but many companies will have strategic concerns in this area as well. Reporting of mesh-related adverse events has reasoned exponentially in the last few years [3], presumably resulting from increasing use and increasing awareness of potential problems. Self-reported complications to the FDA’s MAUDE database have risen for all forms of mesh including mid-urethral tapes. Particularly worrying is the potential that tape-related complications after tape placement can happen many years postoperatively [4].
A key aspect of the litigation relating to mesh use in vaginal prolapse surgery is the lack of premarketing testing of these devices and the weak evidence base [5]. Legal arguments involve the responsibility of the companies to demonstrate safety before marketing, and the urological profession has expressed the desirability of more stringent approaches to the development of surgical devices – especially given the highly stringent requirements for pharmaceutical companies in marketing new drugs. The professionals themselves are not blameless; preoperative counselling on risk, judicious selection of surgery according to the patients’ individual requirements, surgical training, careful follow-up and engagement where problems arise have caused difficulties in many cases previously. These points are expectations of professional practice, and the professions need to adhere to them – if necessary with input from governing bodies to ensure adherence is demonstrable. The area is rapidly changing and we can be sure that substantial dialogue and developments are predictable in the near future.
Approaches to management of tape complications
The management of mesh and tape-related complications is specialised and centralisation of management of these cases appears appropriate in order to have the best chance of acceptable outcome and to develop the necessary skills which would not be possible in centres handling only small numbers of cases. Potential complications include voiding dysfunction, mesh exposure, pain, LUTS, and persisting or recurrent incontinence. Voiding dysfunction is particularly likely if a woman already has a preoperative history of voiding symptoms, previous retropubic surgery, or if other reconstructive procedures are undertaken at the time of tape placement [6]. Voiding dysfunction can occur as a result of urethral compression by the tape – which will usually be palpable as an indentation of the urethra at its midpoint. Alternatively, voiding dysfunction arises from elevation of the endopelvic fascia – in which case, the urethra tends to be drawn upwards towards the retropubic space. In the first case, a tape incision can be effective, but in the latter case, an abdominal procedure to release the endopelvic fascia to its normal configuration might be needed. It is important to avoid instrumenting the urethra and levering the urethra downwards with an instrument placed into the urethra – this carries the risk of crushing the urethra against the tape, and is likely a major potential factor for subsequent erosion into the urethra.
The assessment of women with the tape complication needs to be comprehensive and fastidious. The considerations require awareness of tape exposure, incontinence, voiding dysfunction, proximity of adjacent structures, pain points and the state of the vagina/ labia/ pelvis. If mesh is exposed, it is essential to remove the unwanted material, though it may not be necessary to remove the entire tape. The excision of the material may leave a defect within the urethra, bladder or vagina, which needs to be closed- bearing in mind the principles for avoidance of subsequent fistula formation (i.e. watertight closure and interposition of healthy tissue between repaired structures). The woman will seek continence postoperatively and to deliver this, both the bladder outlet and the reservoir capacity of the bladder will need to be considered. If necessary, the woman may need to self-catheterise afterwards, and whether the patient will find this practical and acceptable must be confirmed preoperatively. The possibility that the planned operation may fail has to be considered, and accordingly steps taken to ensure that subsequent options are not excluded. For example, excision of mesh may best be achieved with placement of a flap of omentum into the area of the defect, to keep open the subsequent possibility of artificial urinary sphincter placement. It is very clear that extensive experience in all aspects of reconstructive urology are needed in order to get the best outcome in this context.
The looming threats
The immediate future sees several key challenges, including:
1. Training of surgeons in primary incontinence surgery to minimise risk of complications arising
2. Training of surgeons to manage complications
3. Regulatory arrangements as authorities come to grips with major complications occurring with significant incidence
4. Strategic concerns as device companies change their view of the merit of this indication for their profitability
5. The need for proper data on device use, outcomes and adverse consequences
6. The ongoing need to find new management options for improving efficacy and safety in surgical management of incontinence
Professional consensus and dialogue is clearly a high priority to ensure a good outcome for all.
Dr Marcus Drake is Consultant Surgeon at the Bristol Urological Institute, Bristol, UK, subspecialising in Female and Reconstructive Urology, Neurourology and Urodynamics He is Chairman of the International Continence Society’s Standardisation Steering Committee
References
1. Novara, G., et al., Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol, 2010. 58(2): p. 218-38.
2. Smith, A.R., W. Artibani, and M.J. Drake, Managing unsatisfactory outcome after mid-urethral tape insertion. Neurourol Urodyn, 2011. 30(5): p. 771-4.
3. Shah, H.N. and G.H. Badlani, Mesh complications in female pelvic floor reconstructive surgery and their management: A systematic review. Indian journal of urology : IJU : journal of the Urological Society of India, 2012. 28(2): p. 129-53.
4. Jones, R., et al., Risk of tape-related complications after TVT is at least 4%. Neurourol Urodyn, 2010. 29(1): p. 40-1.
5. Abrams, P., et al., Synthetic vaginal tapes for stress incontinence: proposals for improved regulation of new devices in Europe. Eur Urol, 2011. 60(6): p. 1207-11.
6. Molden, S., et al., Risk factors leading to midurethral sling revision: a multicenter case-control study. Int Urogynecol J Pelvic Floor Dysfunct, 2010. 21(10): p. 1253-9.
Comments on this blog are now closed.



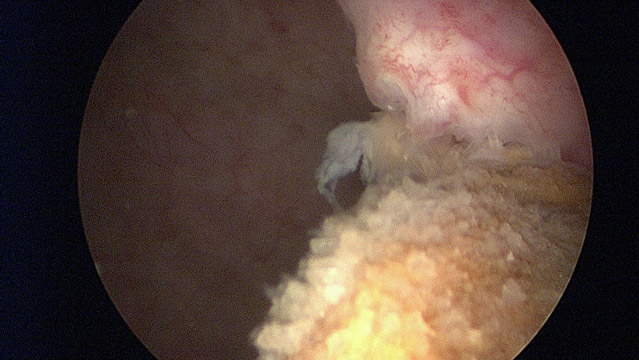
There’s a new and safer way to achieve the same results as Coaptite. Using blood-derived growth factors to treat stress incontinence avoids the risk of granuloma formation.
You can find out more by going to the following website: https://OShot.info/members/stress
There’s a new and safer way to achieve the same results as Coaptite. Using blood-derived growth factors to treat stress incontinence avoids the risk of granuloma formation.
You can find out more by going to the following website: https://www.southernbellemedicine.com/o-shot/