Is Gleason 6 really cancer?
The recently published Viewpoint of the National Cancer Institute working group on “Overdiagnosis and Overtreatment in Cancer” by Esserman and colleagues [1] raises continued discussion as to whether some lesions currently classified as carcinomas should have the designation of “cancer” removed, based on low rates of progression, death, and other adverse outcomes. Pertinent to those interested in urology, a central example in the article is prostatic adenocarcinoma.
One simple answer to this question is that to a small extent, a subgroup of prostatic lesions has already been reclassified as not cancer: In current practice, needle biopsy or radical prostatectomy specimens with an overall Gleason score (GS) of 5 or less are now quite rare in current practice. This shift is due in part to modern updates to the Gleason grading system [2], under which many tumors now reach thresholds for GS6 or above. However, at least some lesions previously considered adenocarcinoma with a low overall GS would now be categorized as atypical adenomatous hyperplasia or adenosis in the era of immunohistochemistry for markers of prostatic basal cells. Nonetheless, the current and more controversial debate surrounds whether some (or all?) tumors currently classified as GS6 could be recategorized as not “cancer”.
Arguments against removing the cancer designation from some prostatic adenocarcinomas:
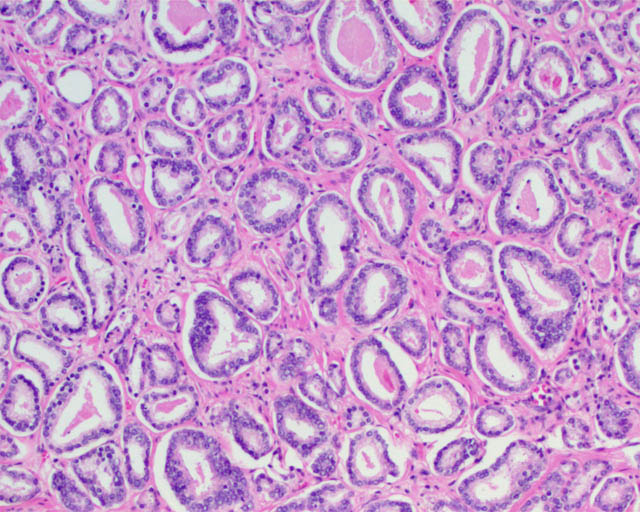
A major difficulty from the pathologic standpoint in adopting a non-cancer nomenclature for some tumors (such as GS6 adenocarcinomas) is that the Gleason pattern 3 component of a GS 3+3=6 tumor (small, round prostatic glands that lack a basal cell layer and infiltrate between benign glands) is for all intents and purposes identical to the Gleason pattern 3 component of a GS 3+4=7 or higher prostate cancer. These similarities are not limited exclusively to the microscopic appearance but also include a number of immunohistochemical and molecular features, as summarized in a recent article addressing this question [3]. Therefore, no pathologic features are as yet defined that ideally predict whether Gleason pattern 3 glands in a biopsy specimen represent a pure GS6 tumor or a component of higher-grade tumor in which the high-grade component is not represented. Not surprisingly, it is not unusual for tumors with GS6 on needle biopsy to be upgraded to GS7 at radical prostatectomy [3], particularly when a high tumor volume is present in the needle biopsy.
Gleason pattern 3 glands from a GS7 tumor, identical to those of a GS6 tumor.
To compare to other cancers with low risk of aggressive behavior, basal cell carcinoma and squamous cell carcinoma of the skin similarly show locally infiltrative properties, supporting their classification as carcinomas by a classical pathologic definition. Despite that the word “carcinoma” continues to be used for these tumors, most patients are not concerned that they have a life-threatening disease and these lesions are even excluded from the American Cancer Society statistics regarding cancers [4]. In the same way, Gleason pattern 3 glands exhibit infiltrative growth by extending between benign glands, invading nerves, and sometimes extending outside of the prostate. This difference in mindset regarding some types of “cancers” could be considered supportive evidence for the assertion in the recent Melbourne Consensus Statement that uncoupling prostate cancer diagnosis from intervention may be more appropriate than removing its “cancer” nomenclature.
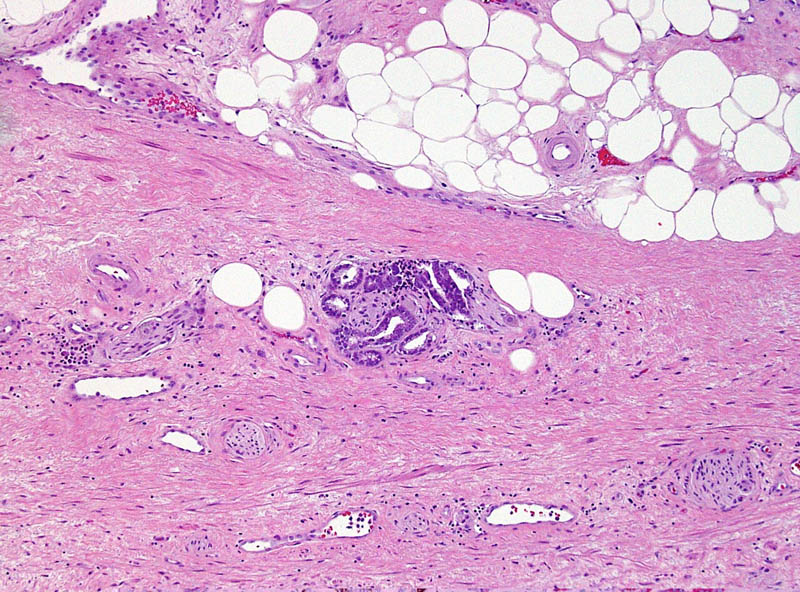
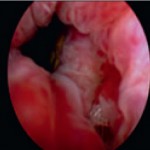
This small GS6 adenocarcinoma was an incidental finding in a radical cystoprostatectomy specimen for bladder cancer but surprisingly extended into periprostatic fat via this focus of perineural invasion.
Supporting removal of the cancer designation from some prostatic adenocarcinomas:
A valid argument of the NCI Viewpoint is that a neoplasm should have a substantive rate of progression and patient death if it is to be considered a cancer. Likewise, others have questioned whether low-volume GS6 tumors fulfill other molecular and pathogenetic hallmarks of cancer, such as unlimited replicative potential and other features [5].
In general, benign and malignant neoplasms can be regarded as having some prototypical gross and microscopic pathologic characteristics, such as a circumscribed vs infiltrative growth and homogeneous vs pleomorphic cell population. However, differentiating benign from malignant lesions also relies heavily on parameters specific to the organ involved. Clear cell renal cell carcinoma, another genitourinary tract tumor, often does not possess these prototypical features of malignancy. Tumors often form a well-circumscribed mass without an “invasive” growth pattern and they often are composed of a uniform population of cells. However, based on known behavior of these tumors, their status as a malignancy is not in doubt. Conversely, renal oncocytoma is a benign neoplasm that shares some of these general features (a round mass composed of a homogeneous population of renal tubular cells). Occasionally oncocytomas appear infiltrative by extending into the perinephric fat or renal vein, yet their status as benign is also not the subject of debate. If some prostate cancers do not have a substantial likelihood of resulting in progression and death, they may not meet an important criterion for a diagnosis of cancer, despite that other features, such as infiltration of tissues, invasion of nerves, and loss of the basal cell layer are characteristic of a malignant neoplasm.
Since a diagnosis of GS6 by needle biopsy is not always predictive of a radical prostatectomy overall GS6, a major challenge to such an approach would be to determine where such a cutoff could be drawn between “cancer” and “not cancer” [5]. If based on tumor volume, it would be difficult to conceptualize that a small amount of GS6 glands would be regarded as a benign lesion, whereas a large amount of identical glands would represent a malignant lesion. Alternatively, the presence of Gleason pattern 4 could used as the point of differentiation (GS7 or above). In the endometrium, a disorganized proliferation of crowded glands with some cytologic features of cancer is regarded as complex atypical hyperplasia. Diagnosis of adenocarcinoma is then reserved for proliferations with a confluent growth of these glands, similar to the threshold for recognizing a component of cribriform glands as Gleason pattern 4. A limitation to such an approach, however, is that a substantial fraction of patients with a needle biopsy GS6 are upgraded to GS7 at radical prostatectomy, as discussed above. Likewise, the ability to treat and monitor GS6 adenocarcinoma nonsurgically is not quite analogous to that of endometrial hyperplasia.
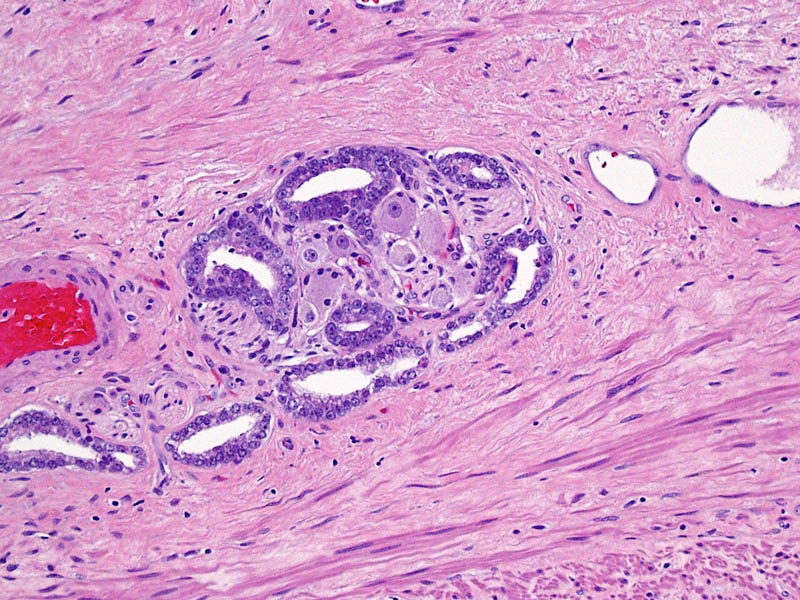
Higher magnification of image 2 shows Gleason pattern 3 glands invading a nerve with ganglion cells.
Other points of discussion
The NCI Viewpoint also suggests that high-grade prostatic intraepithelial neoplasia (HGPIN) no longer be considered cancer or even neoplasia. A comparison to ductal carcinoma in situ (DCIS) of the breast for this argument is somewhat flawed, as HGPIN neither contains the word “carcinoma” nor is justification for treatment in and of itself. Its status as a risk factor for a future cancer even remains debated. The proposal to remove “neoplasia” from HGPIN is also a confusing one, particularly as cervical cancer is noted as an example of the successful application of screening, in which “cervical intraepithelial neoplasia” is the preferred term for precancerous lesions. The authors suggest the designation “indolent lesions of epithelial origin” (IDLE) for cancers in this category to convey their low likelihood of aggressive behavior. However, would recognizing the status of these lesions as at least premalignant neoplasms be more appropriate?
Likely a typographical error in the Viewpoint is that the authors also cite reclassification of urothelial papilloma as papillary urothelial neoplasm of low malignant potential [1]. Since urothelial papilloma has never been considered a malignant neoplasm, the authors likely meant reclassifying “grade 1 urothelial carcinoma” to papillary urothelial neoplasm of low malignant potential.
References
[1] Esserman LJ, Thompson IM, Reid B. Overdiagnosis and Overtreatment in Cancer: An Opportunity for Improvement. JAMA. 2013 Jul 29:
[2] Epstein JI, Allsbrook WC, Jr., Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005 Sep: 29:1228-42
[3] Carter HB, Partin AW, Walsh PC, et al. Gleason score 6 adenocarcinoma: should it be labeled as cancer? J Clin Oncol. 2012 Dec 10: 30:4294-6
[4] Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013 Jan: 63:11-30
[5] Ahmed HU, Arya M, Freeman A, Emberton M. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol. 2012 Nov: 13:e509-17
Sean Williamson is Senior Staff Pathologist in the Department of Pathology and Laboratory Medicine, Henry Ford Health System, Detroit MI, USA. @Williamson_SR



My two concerns with the downgrading of Gleason 3+3 cancer to benign disease are 1) biopsies often underestimate the grade and volume of the tumour. As we are seeing with patients on Active Surveillance, a ‘pussycat’ Gleason 6 not infrequently becomes a much more worrisome tumour (as discussed extremely well in the blog) on repeat biopsy.
2) as we have all seen in patients who have had radiotherapy (or even surgery), patients with Gleason 6 can (and do) turn up a number of years later with metastatic disease, probably due to understaging/under grading at time of diagnosis. Until our methods of diagnosis are more accurate, this will always remain our stumbling point.
Perhaps it is time to redefine Gleason 1 and 2, so that they can be diagnosed on a needle biopsy. It seems to me that the truncation (although correct) of a system which allows grading from 1+1=2 up to 5+5=10 to a system which in reality only allows grading from Gleason 6 to 10, with 6 being borderline benign (as per some opinions), but Gleason 7 being considered lethal, with extended lymph node dissection and adjuvant radiation therapy being recommended as treatment, has the potential to be very problematic. This large jump in potential lethality inherently feels incorrect to me. Perhaps our difficulty is that there is too much heterogeneity in the group we now classify as Gleason 3+3=6, and this is where the division between ‘pussycat’ prostate cancer, and ‘tiger’ prostate cancer should be pursued more vigorously.
Although not based on epidemiological studies, I dread the day that I have to tell a patient that he has metastatic prostate cancer, and needs chemical castration, having told him a number of years earlier that the Gleason 6 disease I found was not defined as cancer any more, and he had nothing to worry about!
Technically speaking, Gleason 6 is cancer. However…
We are currently faced with the potential of losing PSA testing as a means of finding aggressive prostate cancers while still curable. The strongest argument against PSA testing is the gross overtreatment of harmless low risk disease that has gone on and continues to go on. So if a way of stopping this overtreatment is to re-label Gleason 6 as benign disease, or preferably as pre-malignant, then I am strongly in favour.
Concerns over upgrading at radical prostatectomy are overstated. The vast majority of upgrades are to 3+4=7, many of which are also likely to be harmless. There are indeed upgrades to high risk disease also, which are truly concerning. But these are a result of inadequate biopsy, rather than the finding of Gleason 6 on biopsy. Our biopsy techniques clearly need refinement, which is now occurring through multiparametric MRI and transperineal biopsy – refinements that are likely to become standard of care in the years ahead.
So I’d argue that if you can’t find Gleason 7 or higher on a biopsy (unless it’s clearly high volume Gleason 6), don’t undertake curative treatment. If you’re still worried your patient is harbouring something sinister, get an MRI with an expert radiologist and do a transperineal biopsy. Better still, do this first and save your patient the inaccuracies of a standard TRUS biopsy, along with the risk of septicaemia ( – far more life-threatening than Gleason 6 disease!)
In calling Gleason 6 pre-malignant, I would argue that we are far more likely to succeed in undertaking active surveillance in the vast majority of these low risk patients. If we do, we strengthen the argument to continue PSA testing, so that we can continue to find and treat those men who do truly have harmful disease.
(Sorry DBH, I disagree!)
As a pathologist, I would strongly argue for sticking with clear objective disease terminology. At the risk of sounding dogmatic, I believe invasion is cancer.
My arguments against a change include;
– Altering disease definitions to assist clinical/patient decision making is adding an unnecessary step……..why not just improve clinical/patient decision making.
– Patients cope with the diagnosis of cancer in other organs (see above examples of basal cell carcinoma and squamous cell carcinoma), based predominantly on the confidence with which clinicians can tell them that these diagnoses have negligible/very low risk of metastasis respectively; the aim should to be to improve diagnostic/prognostic testing in prostate cancer (Phi test, mpMRI, transperineal biopsies, biomarkers, nomograms) so that clinicians can be equally confident in expressing prognosis to their patients with low grade prostate cancer.
– Proponents of change in terminology should be reminded that an equally confusing, and possibly more litigious situation will arise when trying to explain to a patients how their “pre-malignant” prostate cancer has progressed to higher grade or even metastasised (Dr Chris Hovens presented such a case of ‘metastatic Gleason 6 prostate cancer’ at the recent Melbourne Congress).
– Altering ‘post-diagnosis’ terminology adds another potentially subjective step to a process that is already fraught with interobserver variability; the diagnosis of carcinoma is often hard enough without then having to decide what to call it based on clinician preference (it is my assumption that some clinicians will want to stick to traditional terminology).
– A change like this will add to an already confusing uropathology lexicon; we let you have non-invasive bladder lesion called ‘cancer’ (TCC/papillary urothelial carcinoma) but you’re pushing it with invasive prostate lesion called ‘pre-malignant’.
– That point also reminds me, patients seem to cope with non-surgical treatment for bladder cancer. Maybe it is the complete lack of any intervention that is the problem in low grade prostate cancer.
Don’t get me wrong, I’m all for improved risk stratification in prostate cancer but I believe the bulk of the work needs to be done in the pre-biopsy and post-biopsy stages with improved patient selection, more accurate sampling techniques, better risk stratification and improved patient (and population) education.
Dr Williamson should be congratulated for initiating this debate. I would like to raise a few queries that may further fuel the debate.
1. What is the inter- and intra- observer variation with regards to Gleason 6 cancers?
2. We do know from the older series that radical prostatectomies was performed and indeed external beam radiotherapy were delivered for Gleason 1 and 2 cancers. According to the current consensus, was it really cancer that was treated?! The reason why I raise this is whether we may be discussing in a decade’s time that we treated Gl 3 cancer (that would be branded not cancerous)?!
I strongly support the views of Dr Ryan about the medico-legal implications of re-branding the terminology. What is important is the need for better counselling skills by the physicians in reassuring the patient.
Warm Regards,
The suggestion that a Gleason Score (GS) of 6 is not a cancer would be a dramatic setback to a profession where progress has been limited in recent decades. Making such a change while inappropriate in my professional opinion, would also confuse the non-medical unwitting public at large. Presently men with a GS of 6 have a chance to avoid significant side effects by avoiding caustic radiation or radical surgery while learning to live with prostate cancer much like patients live with Diabetes. It is well noted that dietary and nutritional lifestyle changes assist an active surveillance (AS) or chronic disease management (CDM) protocol benefitting the patient. On the other hand, many men with a GS of 6 have significant cancer where a progressively rising Prostate Specific Antigen (PSA) value signals a much more aggressive cancer than that predicted by the designation of a GS 6. These are cancers that should be treated as they put patient lives in jeopardy. Michael Barry, M.D. from Harvard University and others have suggested that 30-56% of men diagnosed with prostate cancer (likely representing a GS of 6) are over-treated and therefore should be offered a chance to live with their disease! Unfortunately a biopsy based diagnosis without adequate imaging cannot identify the population who should be able to avoid definitive treatment by virtue of minimal disease. To be sure, biopsies often miss cancer altogether even with a rising PSA. Therefore, a GS 6 cancer should not lose its cancer identity or designation but moreover magnetic resonance imaging (MRI) is the only way to state unequivocally the extent of disease allowing men improved confidence in avoiding unnecessary treatment while protecting quality of life altering issues like impotency and incontinence commonly experienced when aggressive treatments like radiation and radical prostatectomy are performed. We owe to our patients to avoid unnecessary confusion in an otherwise contentious discussion arena. Changing the cancer nomenclature does just that; so therefore it should be avoided. Respectfully submitted.
Outstanding review by Dr. WIlliamson. Agrees with (and pre-empts) much of what Dr. Epstein and I discuss in our new article at Urologic Clinics (https://www.urologic.theclinics.com/inpress). I think our main additional contributions are a more detailed critical assessment of the “Hallmarks of cancer,” based on an article by Yuri Lazebnik (https://www.nature.com/nrc/journal/v10/n4/full/nrc2827.html), and the new Gleason categories proposed by Pierorazio and colleagues (https://www.ncbi.nlm.nih.gov/pubmed/23464824).
Outstanding comments as well. I wish this blog were indexed on PubMed, I would have loved to see it and cite it in our article!
This is a great debate but it does make it harder when you are reading this and your husband is diagnosed with Gleason 6 by TRUS biopsy that is high volume and found in every lobe. He also has a history of bladder cancer so radiation is not a very attractive option. Are there good resources to look for more comprehensive information that will help with decision making?