In search of the ROSETTA stone (again)?
We are having an amazing year of scientific discovery in our specialty. 2016 has already seen the results of the only randomised trial comparing open versus robotic radical prostatectomy from Australia and the ProtecT trial from UK discussed intensively on Blogs@BJUI. The PROMIS of MRI is expected to change the practice of prostate biopsies in response to a raised PSA. The teams completing these trials deserve our heartiest congratulations as it is well known how difficult randomised trials in surgery are to initiate and complete.
As if this was not enough, this month the randomised controlled trial comparing Botox (Onabotulinum toxin A) to Interstim (sacral neuromodulation) in patients with refractory overactive bladder has been reported in JAMA. It is otherwise known as the ROSETTA study (Refractory Overactive Bladder: Sacral NEuromodulation v. BoTulinum Toxin Assessment).
This is an example of what collaboration between individuals and teams within a pelvic floor group can achieve. Cindy Amundsen, the lead author, presented the trial results at the #AUA16 late breaking abstract session in San Diego.
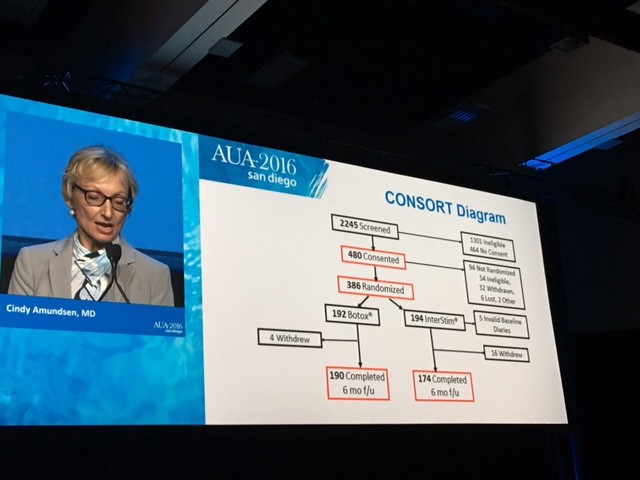
The CONSORT diagram is shown here
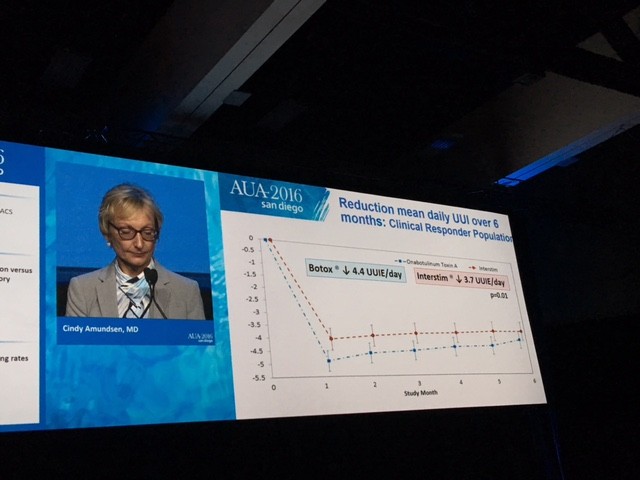
The primary outcome measure showing Botox winning over Interstim (narrowly) in reducing urgency urinary incontinence is demonstrated in this diagram.
The summary results are shown here
So what would you do for your patient with refractory overactive bladder who has failed Anticholinergics and Mirabegron?
I have spent the last week thinking about the trial results carefully and was asked exactly this question at the International Endourology Forum in China. There are a number of important aspects to consider. The dose of Botox used in the trial was 200 units while the licensed dose is 100 units for overactive bladder of non-neurogenic origin. We know that one size does not fit all and indeed some patients failing 100 units need higher doses of Botox. It remains unknown as to what would have happened if 100 units of Botox was compared to Interstim as the authors are quite guarded about their own conclusions about the benefits.
The side effects also need to be carefully discussed with the patient. The UTI rate in the Botox group is about three times that of the Interstim group. Most patients may accept a period of oral antibiotics to counter this. The risk of CISC dropped from 8% at 1 month to 2% at 6 months in the Botox group. This is lower than previously reported in Phase lll studies. The need for revision or removal in the Interstim patients was around 3% – small but not to be ignored.
Punchline
If I was the patient in question, I would have Botox initially, preserving Interstim for later. It is less invasive and can be repeated roughly once a year if needed. Call me “lilly livered” but I do not like the idea of having a little box, however tiny, inside my bum and occasionally having to sit on it! I look forward to the smarter new generation of minimally invasive or even non-invasive nerve stimulators. But then it would need another randomised trial, many years of unanswered questions, perhaps even wastage of a lot of grant money…………..yawn!!
In the meantime, I will take my chances with Botox and counsel my patients accordingly. Unlike the famous ROSETTA stone, the key to understanding the mystery behind hieroglyphs and the controversy as to whether it should at all be in the British museum, I fail to see any such controversy with this nice trial in JAMA.
My thoughts and message are clear. Are yours?




Rosetta is indeed a landmark paper. The first ever RCT to compare BTX to SNS. The authors state 200 U was used for better duration of effect as the main outcome assessed UUI over a 6 month period. There are publications to support this statement. Our own unit presented at BAUS this year comparing 200 (old) vs 100 (new) dosing. Urinary frequency reduction and duration of effect was better with 200 U [ Eldred-Evans et al., P5-16 Journal of Clinical Urology June 2016 vol. 9 no. 1 suppl pp 41]. However 100 U is the licensed dose currently for OnabotulinumtoxinA. So is the study relevant to current ‘real life’ practice?
To me the UUI difference is not huge and many of the QoL measure were non significant between the 2 treatments. Dry rates are important to understand and in this study was 20% vs 2% at 6 months in favour of BTX. The SNS dry rate appears lower than one would expect. 84% had a response in the test phase of SNS using the tined lead and 83% were responders to BTX. This tells me that both are good treatments. SNS is performed typically under sedation or light anaesthesia if using the tined lead. PNE is often done in outpatients. However the tined lead leads to better permanent implant rates. It is minimally invasive. One could argue a young man (The Rosetta study was exclusively in women ) may find a flexible cystoscope and 20 injections in the bladder more invasive than SNS. A recent post FDA approval trial (Insite) for SNS has just reported their 3 year outcome data (Siegel et al., Urology 2016). Implant site pain was 13% which is not insignificant but did not necessarily equate to explantation. If you position the battery well and assess patients sitting prior to implantation you should not be sitting on the Implantable pulse generator !
NICE state BTX should be offered first unless the patient does not want to or cannot do CISC. Other guidelines e.g. EAU offer both treatments side by side. In the UK access to SNS has been an issue but with new NHS England commissioning, one would hope, becomes easier.
Costing is important. But the problem with much of the literature is that it is industry sponsored. Models can be manipulated dependent on what you feed in. In general BTX is more cost effective in the short term and SNS becomes a cost effective option down the line.
Rosetta will report again with longer term follow up which will be important. Of interest to scientists is the urine biomarker work carried out with this study. At the ICS meeting in Tokyo (Richter et al., abstract no.105) data was presented on first morning urines from trial participants with UUI compared to matched controls. Higher baseline IL-8 and CGRP were independently associated with less reduction in UUI and higher CGRP with less reduction in OAB symptom bother at 6 months. This indicated that baseline CGRP maybe predictive of 6 month outcomes. Increases in IL-8 and MMP-9 with decreases in collagenase were also seen in cases.
The Rosetta study would suggest there is an advantage in terms of UUI albeit minimal and perhaps clinically insignificant. UTI is a problem with BTX and further research to understand this is urgently needed. I am not sure if NICE will change their stance at their next review but I like the idea of offering both treatments and letting the patient decide. We may not have all the answers to why patients suffer with iOAB but we have good options in treating refractory symptoms. We have a very good idea of efficacy and adverse events with both treatments and patients can be counselled appropriately.
My punchline: they both work, let the patient decide.
Thank you Arun.
Letting patients decide after a randomised trial, seems to say that we are none the wiser despite the trial. I would be rather concerned if I was the patient being counselled.
The more important question perhaps is what “trade off” patients are willing to accept in order to achieve continence?