Give the pill, or not give the pill. SUSPEND tries to end the debate
June 2015 #UROJC Summary
News of a landmark paper on medical expulsive therapy (MET) for ureteric colic swirled through the convention halls on the last day of the American Urological Association’s Annual Meeting in New Orleans, Louisiana. I watched the Twitter feeds evolve from my desk at home: the first tweets just mentioned the title, then the conclusion, followed by snippets about the abstract. As time passed and people had time to read the manuscript, discussion escalated. Without data to prove it, there seemed to be more Twitter chatter about the SUSPEND trial, even among conference attendees, than the actual AUA sessions.
Robert Pickard and Samuel McClinton’s group utilized a “real-world” study design to publish what many urologists consider to be the “best data” on MET. The study (SUSPEND) randomized 1167 participants with a single 1-10 mm calculi in the proximal, mid, or distal ureter across 24 UK hospitals to 1:1:1 MET with daily tamsulosin 0.4 mg, nifedipine 30 mg, or placebo. The study’s primary outcome was the need for intervention at 4 weeks after randomization. Secondary outcomes assessed via follow-up surveys were analgesic use, pain, and time to stone passage. Though the outcomes were evaluated at 4 weeks after randomization, patients were followed out to 12 weeks.
Some of the study design minutiae are worth specific mention before discussing the results and #urojc chat:
- Treatment allotment was robustly blinded. Participants were handed 28 days of unmarked over-encapsulated medication by sources uninvolved in the remaining portions of the study
- Medication compliance was not verified
- The study protocol didn’t mandate additional imaging or tests at any point
- Participants weren’t asked to strain their urine
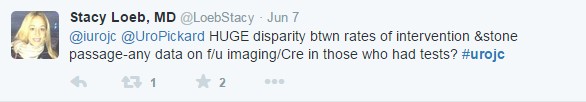
- Secondary outcomes assessed by follow-up surveys were incomplete: 62 and 49% of participants completed the 4- and 12-week questionnaires, respectively
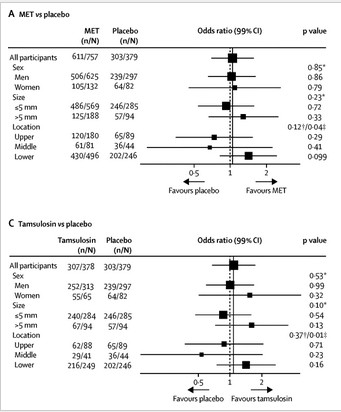
The groups were well balanced, and the results were nullifying. A similar percentage of tamsulosin- , nifedipine-, and placebo-group patients did not require intervention (81%, 80%, and 80%, respectively). A similar percentage of tamsulosin-, nifedipine-, and placebo-group participants had interventions planned at 12 weeks (7%, 6%, and 8%). There were no differences in secondary outcomes, including stone passage. There was a trend toward significance for MET, specifically with tamsulosin, in women, calculi >5 mm, and calculi located in the lower ureter (see image taken from Figure 2).
The authors concluded their paper was iron-clad with results that don’t need replication.
“Our judgment is that the results of our trial provide conclusive evidence that the effect of both tamsulosin and nifedipine in increasing the likelihood of stone passage as measured by the need for intervention is close to zero. Our trial results suggest that these drugs, with a 30-day cost of about US$20 (£13; €18), should not be offered to patients with ureteric colic managed expectantly, giving providers of health care an opportunity to reallocate resources elsewhere. The precision of our result, ruling out any clinically meaningful benefit, suggests that further trials involving these agents for increasing spontaneous stone passage rates will be futile. Additionally, subgroup analyses did not suggest any patient or stone characteristics predictive of benefit from MET.”
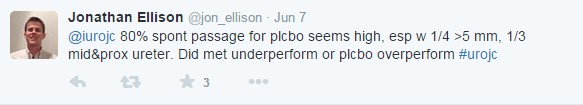
Much of the early discussion focused on the trend toward benefit for MET in cases of calculi >5 mm in the distal ureter:
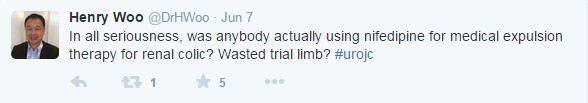
Journal Club participants raised eyebrows to the use of nifedipine and placebo medication in the trial:
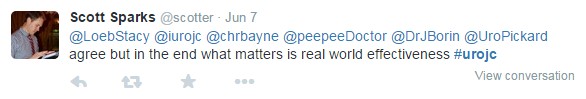
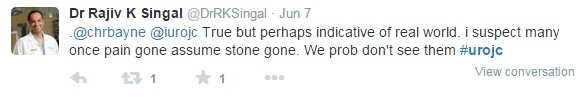
A few hours in, discussion shifted toward the study design, particularly the primary endpoint of absence of intervention at 4 weeks rather than stone passage or radiographic endpoints. The overall consensus was that that this study was a microcosm of “real world” patient care with direct implications for emergency physicians, primary physicians, and urologists.
The $20 question (cost of 4 weeks of tamsulosin according to SUSPEND) is whether or not the trial will change urologists’ practice patterns. Perhaps not surprisingly, opinions differed between American and European urologists.
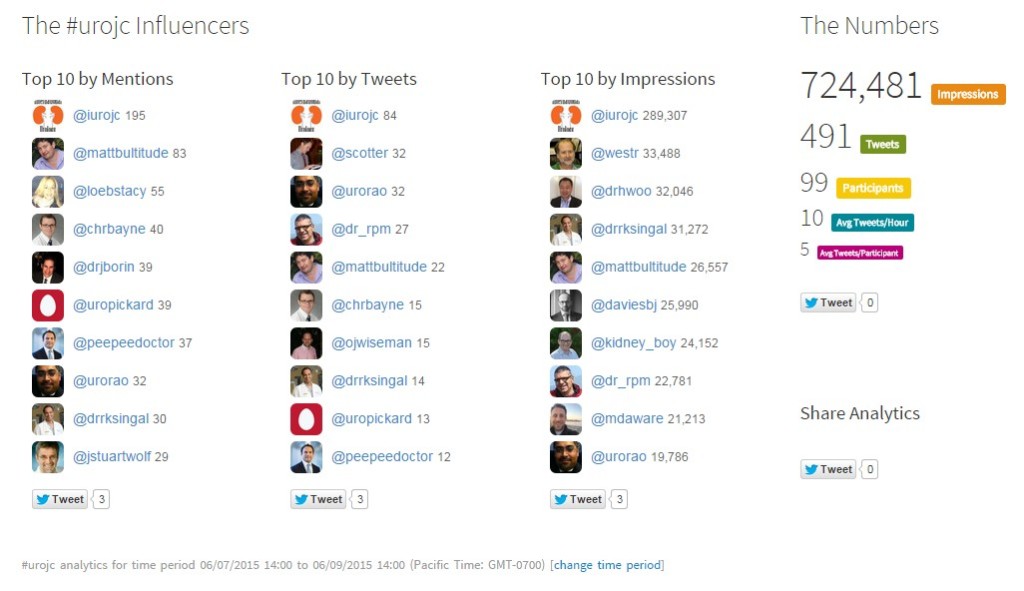
We owe SUSPEND authors Robert Pickard and Sam McClinton special thanks for their availability during the discussion. In the end, the #urojc banter for June 2015 was the largest and most-interactive monthly installment of International Urology Journal Club to date.
Christopher Bayne is a PGY-4 urology resident at The George Washington University Hospital in Washington, DC and tweets @chrbayne.






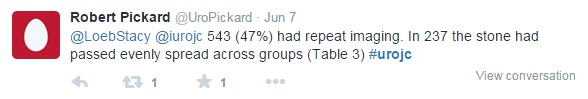




Chris
Great summary and has been a very interesting paper. It creates a fairly unique situation – a fairly simple cheap treatment has become standard practice based on fairly poor evidence but a meta-analysis that shows clear benefit. Then comes along a massive trial that dwarfs the meta-analysis. Which do you believe? What I am fairly sure of is that had this trial been released in 2005, then medical expulsive therapy would not have happened.
For people who are unsure I really do recommend reading the meta-analysis again and remembering just how poor the evidence is. I was shocked when I read it … you just don’t get that info from the abstract.
For me I do believe this trial but have still discussed alpha blockers with some patients since its release. It just takes a lot longer now… telling them there has just been a big trial and it may not work. #confused. The last one I saw took it anyway and stopped it due to dizziness …. Side-effects; off licence and without evidence. I suspect the days of MET are numbered.