Editorial: Role of systematic biopsy in the era of mpMRI and US fusion guidance
The success of multiparametric MRI (mpMRI) and MRI/ultrasound (US) fusion-guided biopsies in improving the detection of prostate cancer in patients with occult disease (elevated PSA level with prior negative biopsies) and optimising the detection of clinically significant cancer has been reported by centres that have served as early adopters of these techniques [1, 2]. Technological advances in MRI and associated imaging protocols, as well as increased clinical experience with MRI interpretation have led to increased prospective detection and characterisation of clinically significant prostate cancer. This, in conjunction with increasing experience with MRI/US fusion-guided prostate biopsy techniques, has led to the re-evaluation of the contributory role and utility of systematic template US-guided prostate biopsies in the diagnosis of prostate cancer. It is an attractive proposition to forego the systematic biopsy when performing MRI-directed fusion biopsy, as this would minimise the duration, morbidity, and overall cost of the biopsy procedure and post-biopsy pathology processing. However, before adopting this approach, it is important to first consider the potential possibility of missing clinically significant cancer diagnoses when relying on the targeted biopsy cores in isolation.
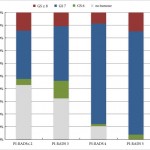
In this issue of BJUI, Borkowetz et al. [3] report their results of biopsy histological yields on systematic biopsies compared with MRI/US fusion biopsies in their series of patients who underwent radical prostatectomy (RP). These results corroborate previously reported comparisons of fusion biopsy of suspicious lesions on MRI performed concurrently with systematic biopsy, consistently showing an improved detection of both overall prostate cancer foci and, more importantly, an improved detection of clinically significant higher grade cancer foci [1, 2]. It is important to note that the overall detection rate and detection rate for clinically significant prostate cancer was highest when fusion and systematic biopsies were evaluated in conjunction with each other. Another important factor to consider when evaluating the utility and value of these biopsy techniques is the concordance of the pathology of the biopsy specimen with the final pathology of the RP specimen, the ‘gold standard’. The concordance of Gleason grade assigned on targeted fusion-biopsy cores and RP outperformed that of systematic biopsy cores and RP. This, in essence, suggests that targeted biopsy can perform as well, and likely better, than the systematic biopsy approach of sampling the prostate with a systematic-sextant approach, which has been the long standing standard of care for the diagnosis of prostate cancer. Again, it is important to note that the greatest concordance in this study was achieved when the results of the fusion and systematic biopsy cores were combined.
The question now arises regarding the ‘cost’ for the incremental improvement in cancer detection provided by the combination of both MRI-directed fusion biopsy and the systematic biopsy approach. The improved negative predictive value parallels the increased sensitivity for cancer detection by having a larger sampling of the prostate by augmenting the number of biopsy cores sampled and submitted for histopathological evaluation. The area under the curve for detection of clinically significant cancer reported by Borkowetz et al. [3] was not improved by adding systematic biopsies to the targeted biopsies. However, this experience described a mixed population of patients, most of whom had undergone prior prostate biopsy with benign pathology. This creates an enriched population who likely harbours prostate cancers that are more occult to the systematic biopsy approach, thus improving the diagnostic yield of MRI-directed biopsies even further. This is concordant with the work presented by Mendhiratta et al. [4], where systematic biopsies added little to the diagnosis of clinically significant prostate cancer in a population of men undergoing MRI/US fusion-guided biopsy after prior cancer-negative biopsy sessions.
Alternatively, current datasets for biopsy naïve patients have not shown the same degree of convincingly improved detection with targeted biopsies over systematic biopsies. In fact, Delongchamps et al. [5] recently reported a slightly lower rate of overall cancer detection with fusion-guided targeted biopsies vs systematic biopsy cores; however, the difference in detection of clinically significant prostate cancer was not statistically significant. Further study of the role of targeted biopsy in the biopsy naïve patient population is warranted, as there is suggestion that cancer detection efficiency per needle core is significantly improved with MRI-directed biopsies over systematic biopsies [6]. Alternatively, in patients with prior negative systematic biopsies and continued clinical suspicion for prostate cancer, a repeat biopsy session with targeted cores alone may be appropriate, particularly as these patients have previously undergone standard-of-care, extended sextant biopsy.