Editorial: Expanding the feasibility of nephron‐sparing surgery: time for a paradigm shift?
With the rapid implementation of ‘targeted’ therapies, kidney cancer has entered a new era where old paradigms are being challenged, and new ones can be explored. The idea of delivering ‘neoadjuvant’ systemic therapy to alter the surgical treatment of advanced RCC was suggested in this same journal ~10 years ago as a proof‐of‐concept study [1]. Since then, a plethora of small case series has investigated the safety and feasibility of different targeted agents in the preoperative setting to facilitate surgical resection of locally advanced disease, mostly with a ‘cytoreductive’ (rather than ‘curative’) intent.
In this issue of the BJU Int, Lebacle et al. [2] evaluated the role of neoadjuvant axitinib, an oral tyrosine kinase inhibitor currently recommended as a second‐line option for metastatic clear cell RCC, to downstage cT2 kidney cancer and allow a partial nephrectomy (PN). In this multicentre prospective study, 18 patients with RCC (median tumour size 7.6 cm and R.E.N.A.L. [Radius; Exophytic/Endophytic; Nearness; Anterior/Posterior; Location] score 11) were enrolled. A median tumour size reduction of 17% was obtained, and the primary outcome (‘clinical downstaging’ to cT1 to allow PN) was achieved in 12 patients (67%). Overall, 16 patients underwent PN, as this was successfully done also in four of six (67%) patients who were not ‘down‐staged’ by the drug. Notably, about half of the PNs were performed with a robotic approach. Whilst axitinib was well tolerated, five patients experienced a high‐grade complication after surgery, including one death. Interestingly, final pathology showed upstaging to pT3a disease in seven patients, and two positive margins. Moreover, about a third of patients had metastatic progression and two had recurrence at 2 years. Thus, while the authors noted axitinib to be effective in reducing tumour size and achieving a clinical downstaging in most patients, the significant presence of pT3a disease calls into question the overall efficacy (to truly pathologically downstage) or desirability (most of the tumours that were not downstaged still successfully underwent PN) of the study’s main stated aim.
The rapid adoption of robotic surgery and the increasing experience with PN techniques translated into expanding indications for minimally invasive nephron‐sparing surgery (NSS), to include also T1b and T2 renal masses [3], and the field is primed for a possible paradigm shift. Whether or not a PN is doable, regardless of the technique, remains in the hands of the surgeon, who makes that decision based on previous personal experience. This is also the case for the present study, where the primary outcome was simply represented by the number of patients who could get a PN (instead of a radical nephrectomy). As such, is such a subjective endpoint (feasibility of PN) clinically meaningful? While disagreement may occur over the risk of PN in complex and elective cases, the desirability of nephron preservation in imperative and most elective circumstances is supported by evidence that largely suggests that PN translates into better renal function. In addition, recent findings suggest that estimated GFR preservation might translate into better cancer‐specific survival [4]. Certainly, this type of endpoint (whether a PN is feasible) is prone to intrinsic bias and limitations.
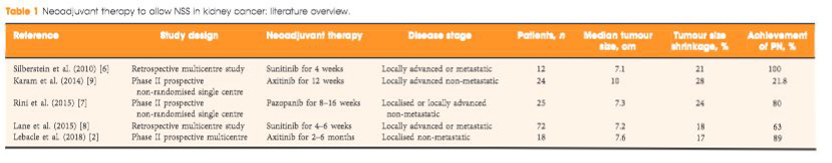
Only a limited number of studies have specifically explored the role of neoadjuvant therapy to enable NSS with variable results [5] (Table 1) [2, 6, 7, 8, 9]. Overall, these studies suggest that even a modest tumour size reduction can facilitate kidney preservation in a significant number of cases. Amongst these studies, only one had assessed axitinib in this specific setting [9]. Differences in outcomes between that trial and the present one by Lebacle et al. [2] could be explained by differences in study populations and/or drug regimens. A more recent study by Karam et al. [10], showed that inter‐observer agreement regarding the feasibility of a PN is quite variable, which is not surprising. For this reason, those authors advocated the need for a ‘resectability score’.
In conclusion, utility of neoadjuvant therapy to modify tumour size and facilitate NSS is an active and exciting area of clinical investigation, fuelled by the rapidly changing landscape of systemic therapies for RCC. It is too early to call for a paradigm shift, but a few ongoing studies might provide some meaningful answers soon. Amongst these, the PADRES (Prior Axitinib as a Determinant of Outcome of REnal Surgery) is an ongoing North American multicentre phase II study of axitinib with the aim of recruiting 50 patients [5]. While waiting for more robust evidence, the use of neoadjuvant therapy to facilitate NSS should still be deemed as investigational.
References
- , , et al. Neoadjuvant targeted therapy and advanced kidney cancer: observations and implications for a new treatment paradigm. BJU Int 2008; 102: 692– 6
- , , et al. Evaluation of axitinib to downstage cT2a renal tumours and allow partial nephrectomy: a phase II study. BJU Int 2019; 123: 804– 10
- , , et al. Outcomes of robot‐assisted partial nephrectomy for clinical T2 renal tumors: a multicenter analysis (ROSULA Collaborative Group). Eur Urol 2018; 74:226– 32
- , , et al. Below safety limits, every unit of glomerular filtration rate counts: assessing the relationship between renal function and cancer‐specific mortality in renal cell carcinoma. Eur Urol 2018; 74: 661– 7
- , , et al. Neoadjuvant therapy for localized and locally advanced renal cell carcinoma. Urol Oncol 2018; 36: 31– 7
- , , et al. Feasibility and efficacy of neoadjuvant sunitinib before nephron‐sparing surgery. BJU Int 2010; 106: 1270– 6
- , , et al. A phase II study of pazopanib in patients with localized renal cell carcinoma to optimize preservation of renal parenchyma. J Urol 2015; 194: 297– 303
- , , et al. Presurgical sunitinib reduces tumor size and may facilitate partial nephrectomy in patients with renal cell carcinoma. Urol Oncol 2015; 33: 112.e15–21.
- , , et al. Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur Urol 2014; 66: 874– 80
- , , et al. Variability of inter‐observer agreement on feasibility of partial nephrectomy before and after neoadjuvant axitinib for locally advanced renal cell carcinoma (RCC): independent analysis from a phase II trial. BJU Int 2016; 117: 629– 35