Editorial: Cabazitaxel for the therapy of mCRPC in the aftermath of CHAARTED
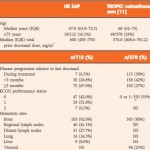
In this issue of BJUI, Bahl et al. [1] describe clinical outcomes amongst 112 patients with metastatic castration-resistant prostate cancer (mCRPC) receiving cabazitaxel 25 mg/m2 in the UK Early Access Programme (EAP). Patients also received daily oral corticosteroids in a fashion consistent with the phase III TROPIC study and had experienced disease progression during or after docetaxel [2]. The study suggests that improved quality of life and only modest toxicity are achieved with cabazitaxel. Moving forward, the key challenge will be translating these data to clinical practice in the context of a rapidly changing therapeutic landscape.
A veritable game of leapfrog has been ongoing in metastatic prostate cancer. In 2010, two agents were approved by the US Food and Drug Administration, sipuleucel-T and cabazitaxel. Sipuleucel-T, a dendritic cell vaccine, remains largely applied in the pre-docetaxel setting in patients who are either asymptomatic or minimally symptomatic. By contrast, the phase III TROPIC trial leading to the approval of cabazitaxel exclusively included patients who had previously received docetaxel. These approvals made for a relatively straightforward approach to mCRPC, with docetaxel therapy flanked by sipuleucel-T and cabazitaxel. Within 2 years, two novel endocrine therapies emerged, abiraterone and enzalutamide, initially approved in the post-docetaxel space and subsequently in the pre-docetaxel space. A fifth agent, radium-223, was approved for mCPRC in 2013 based on a trial conducted in symptomatic patients with bone metastases who were either post-docetaxel or unfit for or refused docetaxel.
Although editorials and position papers abound, there is actually little consensus regarding the sequencing of these agents. Furthermore, the classification of these therapies as pre- or post-docetaxel may be rendered obsolete in the aftermath of the recently reported CHAARTED trial [3]. In that study, a total of 790 patients with mostly extensive (defined as presence of visceral disease or ≥4 bone lesions with ≥1 lesion beyond the spine or pelvis) newly diagnosed metastatic castration-sensitive prostate cancer were randomized to receive either androgen deprivation therapy (ADT) alone or ADT with six cycles of docetaxel (without daily corticosteroids). The study was closed after a planned interim analysis showed a significant survival advantage in the experimental arm; median overall survival was 57.6 months with docetaxel with ADT vs 44.0 months with ADT alone (hazard ratio 0.49, 95% CI 0.37–0.65; P < 0.001). Furthermore, recent data from the phase III STAMPEDE trial corroborate the robust increment provided by combining docetaxel with ADT in patients with metastatic or high-risk non-metastatic castration-sensitive disease [4]. Thus, for many patients, docetaxel may leap to the fore.
If this is the case, where will cabazitaxel be applied? In patients with mCRPC who have received docetaxel in the castration-sensitive setting, either reinstitution of docetaxel or one of the new agents approved since 2010 may be appropriate. The report by Bahl et al. provides useful data to suggest that cabazitaxel would be reasonably tolerated in this setting, and reports quality-of-life benefits in conjunction with a low incidence of neuropathy and no toxic deaths. Conversely, despite the fact that 79.5% of patients received prophylactic G-CSF from cycle 1 and an additional 5.3% received G-CSF with subsequent cycles, 6.3% experienced neutropenic sepsis, which attests to the substantial myelosuppression caused by this agent. The optimum sequencing of all of the available agents for mCRPC is unclear and there is an absence of validated predictive biomarkers to deploy personalized therapy. Hence, eligibility criteria employed in the landmark trials, and clinical factors such as Gleason score and duration of prior ADT and comorbidities have been used to select agents, although these strategies remain unvalidated. There are published retrospective clinical experiences that address sequencing, which are not definitive. Since the advent of abiraterone and enzalutamide, the use of cabazitaxel has declined. Intriguingly, some but not all retrospective studies suggest that cabazitaxel followed by androgen axis inhibitors might lead to improved outcomes compared with androgen axis inhibitors followed by cabazitaxel [5, 6]. Another piece in the puzzle is provided by retrospective studies suggesting that cabazitaxel may retain substantial activity even after docetaxel and novel androgen inhibitors, while docetaxel appears to show poorer activity after androgen inhibitors [7].
In summary, the EAP data from Bahl et al. [1] characterizes the activity and safety of cabazitaxel in a real-world population. Furthermore, the use of prophylactic G-CSF in accordance with guidelines appeared to eliminate the deaths from neutropenic sepsis observed in the TROPIC trial, which did not use routine prophylactic G-CSF. The ongoing three-arm phase III FIRSTANA trial compares cabazitaxel with docetaxel as first-line chemotherapy for mCRPC and also attempts to refine dosing by investigating both the 25 and 20 mg/m2 doses. Similarly, the PROSELICA phase III trial attempts to show the non-inferiority of the 20 mg/m2 dose of cabazitaxel compared with the 25 mg/m2 dose in the post-docetaxel setting. Randomized phase II trials are investigating the impact of early switching of the taxane (docetaxel or cabazitaxel) in the absence of PSA decline ≥30% within 3 months and the impact of switching to cabazitaxel vs a different androgen inhibitor in those progressing on a first-line androgen inhibitor within 6 months.