Article of the Week: Quantifying ATP release from isolated bladder urothelial cells
Every Week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
ATP release from freshly isolated guinea-pig bladder urothelial cells: a quantification and study of the mechanisms involved
OBJECTIVES
To quantify the amount of ATP released from freshly isolated bladder urothelial cells, study its control by intracellular and extracellular calcium and identify the pathways responsible for its release.
MATERIALS AND METHODS
Urothelial cells were isolated from male guinea-pig urinary bladders and stimulated to release ATP by imposition of drag forces by repeated pipetting. ATP was measured using a luciferin-luciferase assay and the effects of modifying internal and external calcium concentration and blockers of potential release pathways studied.
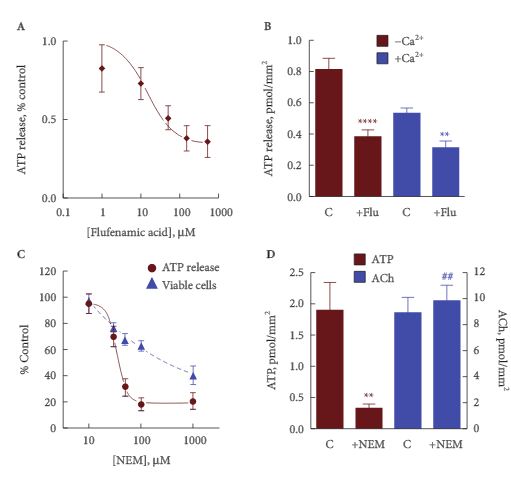
RESULTS
Freshly isolated guinea-pig urothelial cells released ATP at a mean (sem) rate of 1.9 (0.1) pmoles/mm2 cell membrane, corresponding to about 700 pmoles/g of tissue, and about half [49 (6)%, n = 9) of the available cell ATP. This release was reduced to a mean (sem) of 0.46 (0.08) pmoles/mm2 (160 pmoles/g) with 1.8 mm external calcium, and was increased about two-fold by increasing intracellular calcium. The release from umbrella cells was not significantly different from a mixed intermediate and basal cell population, suggesting that all three groups of cells release a similar amount of ATP per unit area. ATP release was reduced by ≈50% by agents that block pannexin and connexin hemichannels. It is suggested that the remainder may involve vesicular release.
CONCLUSIONS
A significant fraction of cellular ATP is released from isolated urothelial cells by imposing drag forces that cause minimal loss of cell viability. This release involves multiple release pathways, including hemichannels and vesicular release.