Article of the Week: Analysis of hydrogel spacer for PCa RT
Every Week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video discussing the paper.
If you only have time to read one article this week, it should be this one.
Prospective analysis of hydrogel spacer for patients with prostate cancer undergoing radiotherapy
Michael Chao*†, Huong Ho† , Yee Chan*‡, Alwin Tan§, Trung Pham¶, Damien Bolton*‡, Andrew Troy*, Catherine Temelcos**, Shomik Sengupta*†† , Kevin McMillan‡, Chee Wee Cham§, Madalena Liu‡, Wei Ding†, Brindha Subramanian†, Jason Wasiak*‡‡, Daryl Lim Joon*†, Sandra Spencer† and Nathan Lawrentschuk*
*The Austin Hospital, Heidelberg, Vic., Australia, †Genesis Cancer Care Victoria, Ringwood East, Vic., Australia, ‡Ringwood Private Hospital, Ringwood East, Vic., Australia, §The Bays Hospital, Mornington, Vic., Australia, ¶ The Valley Private Hospital, Mulgrave, Vic., Australia, **St Vincent’s Hospital, Fitzroy, Vic., Australia, ††Melbourne University; Eastern Health Clinical School, Monash University, Clayton, Vic., Australia, and ‡‡University of Melbourne, Melbourne, Vic., Australia
Abstract
Objective
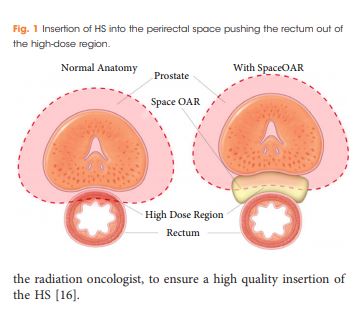
To report on the dosimetric benefits and late toxicity outcomes after injection of hydrogel spacer (HS) between the prostate and rectum for patients treated with prostate radiotherapy (RT).
Patients and Methods
In all, 76 patients with a clinical stage of T1–T3a prostate cancer underwent general anaesthesia for fiducial marker insertion plus injection of the HS into the perirectal space before intensity‐modulated RT (IMRT) or volumetric‐modulated arc RT (VMAT). HS safety, dosimetric benefits, and the immediate‐ to long‐term effects of gastrointestinal (GI) toxicity were assessed.
Results
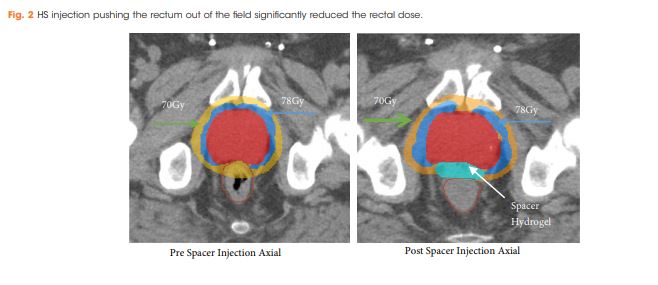
There were no postoperative complications reported. The mean (range) prostate size was 66.0 (25.0–187.0) mm. Rectal dose volume parameters were observed and the volume of rectum receiving 70 Gy (rV70), 75 Gy (rV75) and 78 Gy (rV78) was 7.8%, 3.6% and 0.4%, respectively. In all, 21% of patients (16/76) developed acute Grade 1 GI toxicities, but all were resolved completely by 3 months after treatment; whilst, 3% of patients (2/76) developed late Grade 1 GI toxicities. No patients had acute or late Grade ≥2 GI toxicities.
Conclusion
Injection of HS resulted in a reduction of irradiated rectal dose volumes along with minimal GI toxicities, irrespective of prostate size.