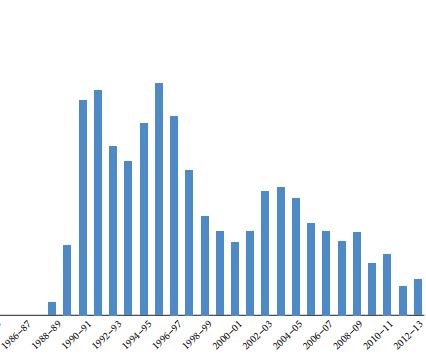
Article of the Month: Is there an anti-androgen withdrawal syndrome with enzalutamide?
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Is there an anti-androgen withdrawal syndrome with enzalutamide?
Alejo Rodriguez-Vida1, Diletta Bianchini2, Mieke Van Hemelrijck3, Simon Hughes1, Zafar Malik4, Thomas Powles5, Amit Bahl6, Sarah Rudman1, Heather Payne7, Johann de Bono2 and Simon Chowdhury1,*
1 Guy’s and St Thomas’ NHS Foundation Trust, Great Maze Pond, London, UK, 2 Royal Marsden NHS Foundation Trust and Institute of Cancer Research, Sutton, UK, 3 King’s College London, Division of Cancer Studies, Cancer Epidemiology Group, London, UK, 4 Clatterbridge Cancer Centre NHS Foundation Trust, Bebington, UK, 5 St. Bartholomew’s Hospital NHS Foundation Trust, London, UK, 6 University Hospitals Bristol NHS Foundation Trust, Bristol, UK, 7 University College Hospital, London, UK
OBJECTIVE
To examine prostate-specific antigen (PSA) levels after enzalutamide discontinuation to assess whether an antiandrogen withdrawal syndrome (AAWS) exists with enzalutamide.
METHODS
We retrospectively identified 30 consecutive patients with metastatic prostate cancer who were treated with enzalutamide after docetaxel. Post-discontinuation PSA results were available for all patients and were determined at 2-weekly intervals until starting further anticancer systemic therapy. PSA withdrawal response was defined as a PSA decline by ≥50% from the last on-treatment PSA, with a confirmed decrease ≥3 weeks later. Patient characteristics were evaluated in relation to the AAWS using univariate logistic regression analysis.
RESULTS
The median (range) patient age was 70.5 (56–86) years and the median (range) follow-up was 9.0 (0.5–16) months. The most common metastatic sites were the bone (86.7%) and lymph nodes (66.7%). Most patients (70%) had previously received abiraterone and 12 patients (40%) had also received cabazitaxel. The median (range) treatment duration with enzalutamide was 3.68 (1.12–21.39) months. PSA levels after enzalutamide withdrawal were monitored for a median (range) time of 35 (10–120) days. Only one patient (3.3%) had a confirmed PSA response ≥50% after enzalutamide discontinuation. One patient (3.3%) had a confirmed PSA response of between 30 and 50% and another patient (3.3%) had an unconfirmed PSA response of between 30 and 50%. The median overall survival was 15.5 months (95% CI 8.1–24.7). None of the factors analysed in the univariate analysis were significant predictors of PSA decline after enzalutamide discontinuation.
CONCLUSIONS
This retrospective study provides the first evidence that enzalutamide may have an AAWS in a minority of patients with metastatic castration-resistant prostate cancer. Further studies are needed to confirm the existence of an enzalutamide AAWS and to assess its relevance in prostate cancer management.