Editorial: Human development and its impact on genitourinary cancers
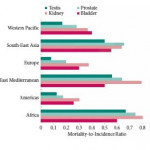
Using the extensive data from the WHO International Agency for Research on Cancer and the United Nations Human Development Report, Greiman et al. [1] aimed to investigate how human development is associated with incidence and mortality of genitourinary cancers. Even though they generate some interesting descriptive findings, we have to remain critical of these descriptive statistics and carefully assess what needs to be investigated next.
Firstly, despite having highlighted the need for attention to indicators of longevity, education, and income per head when assessing human development, the human development index (HDI) is a rather crude measurement. As a geometric mean of normalised indices for each of these three domains, the HDI simplifies but only captures part of what human development entails. Important indicators of health care such as inequalities, poverty, human security, and empowerment are not reflected in the HDI (www.hdr.undp.org). In the context of cancer incidence and mortality this is an important limitation, as it has for instance been shown that socioeconomic status affects early phase cancer trial referrals, which can be considered as a proxy for access to health care [2]. This inequality has been hypothesised to be linked to more comorbidities and lower education in those who are most deprived – a complex interaction which may not be completely captured by the HDI.
Secondly, registration of incidence and mortality of cancers may vary substantially between countries based on both medical practice and governance. These differences are important when trying to generate hypotheses following the ecological study of Greiman et al. [1]. In the case of bladder cancer, for instance, mortality has been estimated to be 17% in the Netherlands, compared to 22% in the USA, and 50% in the UK. As cancer treatments are expected to be similar in these developed countries, it has been thought that a lower registration of non-muscle-invasive bladder cancer in the UK could explain this higher proportion [3]. Thus, discrepancies in cancer registration, even between developed countries, may limit our awareness of cancer burden.
Thirdly, the study design suffers from ‘ecological fallacy’. The latter refers to the inability to draw causal inference about the effect of the HDI on genitourinary cancer at the individual level, in conjunction with the underlying problem of heterogeneity of exposure levels [4]. This limitation was not mentioned by Greiman et al. [1], but affects their conclusions. The lack of information on, for instance, smoking data, comorbidities, and ethnicity make it difficult to understand how development is affecting cancer incidence or mortality. It would have been interesting to also investigate cancers other than genitourinary cancers because a comparison of different tumour types might have shed light on differences in medical practice or risk factors across countries and help tease out the ecological effect of human development.
Despite the aforementioned limitations, the descriptive analysis by Greiman et al. [1] can be helpful for generating hypotheses – as also outlined by the authors. This ecological effect of human development on incidence and mortality rates of genitourinary cancers is particularly relevant when evaluating the impacts of prevention and intervention programmes for these cancers. Their findings suggest that further investigation is required to examine the hypothesis regarding human development and incidence/mortality of genitourinary cancers. To further elucidate this association, methodological challenges will need to be overcome, as HDI assessment has been criticised for being too crude. Nevertheless, it should be possible to collect more detailed information to allow for an understanding of which components of a country’s collective resources affect cancer incidence and mortality the most, e.g. differences in resources used for cancer detection and treatment.