Article of the Week: Complications after serial prostate biopsies in men on AS
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Complications after prostate biopsies in men on active surveillance and its effects on receiving further biopsies in the Prostate cancer Research International: Active Surveillance (PRIAS) study
Leonard P. Bokhorst*, Inari Lepisto†, Yoshiyuki Kakehi‡, Chris H. Bangma*, Tom Pickles§, Riccardo Valdagni¶, Arnout R. Alberts*, Axel Semjonow**, Petra Str
olin††, Manuel F. Montesino‡‡, Viktor Berge§§, Monique J. Roobol* and Antti Rannikko†
*Department of Urology, Erasmus University Medical Center, Rotterdam, The Netherlands,
†Department of Urology, Helsinki University Central Hospital, Helsinki, Finland,
‡ Department of Urology, Kagawa University Faculty of Medicine, Kagawa, Japan,
§Department of Radiation Oncology, British Columbia Cancer Agency, Vancouver, BC, Canada,
¶Prostate Cancer Program and Radiation Oncology 1, Fondazione IRCSS Istituto Nazionale dei Tumori, Milan, Italy,
**Department of Urology, Prostate Center, University Hospital Muenster, Muenster,
††Department of Urology, Martini Klinik, Hamburg, Germany,
‡‡Department of Urology, Hospital Virgen del Camino, Pamplona, Spain, and
§§Department of Urology, Oslo University Hospital, Oslo, Norway
Objective
To study the risk of serial prostate biopsies on complications in men on active surveillance (AS) and determine the effect of complications on receiving further biopsies.
Patients and methods
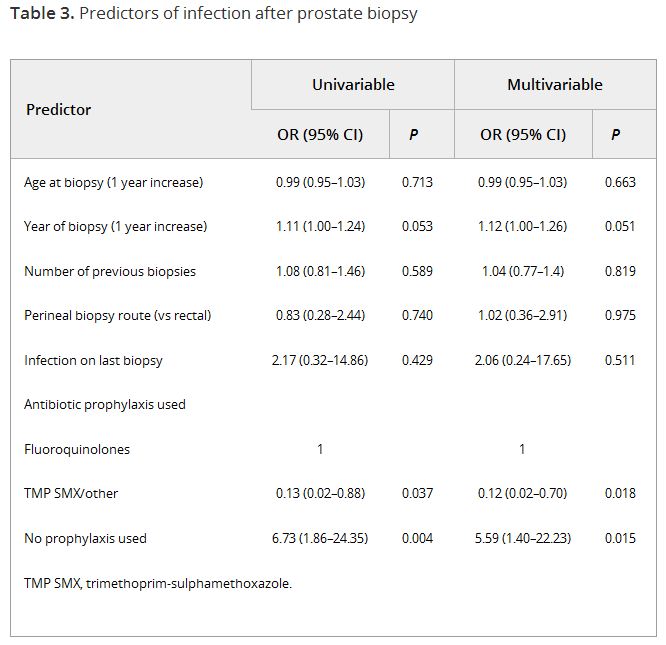
In the global Prostate cancer Research International: Active Surveillance (PRIAS) study, men are prospectively followed on AS and repeat prostate biopsies are scheduled at 1, 4, and 7 years after the diagnostic biopsy, or once yearly if prostate-specific antigen-doubling time is <10 years. Data on complications after biopsy, including infection, haematuria, haematospermia, and pain, were retrospectively collected for all biopsies taken during follow-up in men from several large participating centres. Generalised estimating equations were used to test predictors of infection after biopsy. Competing risk analysis was used to compare the rates of men receiving further biopsies between men with and without previous complications.
Results
In all, 2 184 biopsies were taken in 1 164 men. Infection was reported after 55 biopsies (2.5%), and one in five men reported any form of complication. At multivariable analysis, the number of previous biopsies was not a significant predictor of infection (odds ratio 1.04, 95% confidence interval 0.76–1.43). The only significant predictor for infection was the type of prophylaxis used. Of all men with a complication at the diagnostic or first repeat biopsy, 21% did not have a repeat biopsy at the time a repeat biopsy was scheduled according to protocol, vs 12% for men without a previous biopsy complication.
Conclusion
In our present cohort of men on AS, we found no evidence that repeat prostate biopsy in itself posed a risk of infection. However, complications after biopsy were not uncommon and after a complication men were less likely to have further biopsies. We should aim to safely reduce the amount of repeat biopsies in men on AS.